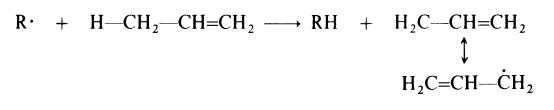Translucent, white solid.Tensile strength 5000 psi, flexural
strength 7000 psi, usable up to 121C. Insoluble
in cold organic solvents; softened by hot solvents.
Maintains strength after repeated flexing. Degraded
by heat and light unless protected by antioxidants.
Readily colored; good electrical resistance; low
water absorption and moisture permeability; poor
impact strength below ?9.4C; not attacked by fungi
or bacteria; resists strong acids and alkalies up to
60C, but is attacked by chlorine, fuming nitric acid,
and other strong oxidizing agents. Combustible, but
slow-burning. Fair abrasion and good heat resis-
tance if properly modified. Can be chrome-plated,
injectionand blow-molded, and extruded.
Polypropylene is a low-density resin that offers a good balance
of thermal, chemical, and electrical properties, along with
moderate strength. Strength can be significantly increased by
using reinforcing agents such as glass fiber. Polypropylene has
limited heat resistance, but it can be used in applications that
must withstand boiling water or steam sterilization.
Polypropylenes can resist chemical attack and are unaffected
by aqueous solutions of inorganic salts or mineral
acids and bases, even at high temperatures. They are not
attacked by most organic chemicals, and there is no solvent
for these resins at room temperature. The resins are attacked,
however, by halogens, fuming nitric acid, other active oxidizing
agents, and by aromatic and chlorinated hydrocarbons
at high temperatures .
Polypropylene is translucent and autoclavable. Properties
can be improved by compounding with fillers, by blending with synthetic elastomers, and by copolymerizing with small
amounts of other monomers.
Modifier for waxes to reduce blocking, scuffing and abrasion. Improves pigment dispersion in polypropylene films and fibers.
Base polymer in hot melt adhesives and paper-laminating, extender and viscosity modifier in caulks and sealants and waterproofing agent in wire and cable applications.
General extrusion grade polymer.
Used with ram- and screw-injection machines. For automotive, housewares, general molding products and multi- and monofiliment fiber.
Polypropylene (PP) is a thermoplastic material used in a wide variety of applications including packaging, labeling, textiles, etc. Due to high processability and low cost, PP is one of the most extensively produced polymers, especially, for auto industry. Pristine PP is resistant to photo-oxidation and thermal oxidation at moderate temperatures. However, PP is sensitive to various external aging environments (such as heat, light, and radiation), and, hence, has a relatively low service temperature.When PP is exposed to high temperatures or to an irradiation environment, the tertiary hydrogen atoms present in PP chains are susceptible to be attacked by oxygen. It is well known that PP oxidation depends on both light and temperature in outdoor aging conditions. PP can also be photo-degraded because several molecular chains are affected in the wavelength range from 310 to 350 nm.
ChEBI: A polymer compose of repeating propane-1,2-diyl units.
polypropylene: Anisotactic polymer existing in bothlow and high formula-weight forms.The lower-formula-weight polymer ismade by passing propene at moderatepressure over a heated phosphoricacid catalyst spread on aninert material at 200°C. The reactionyields the trimer and tetramer. Thehigher-formula-weight polymer isproduced by passing propene into aninert solvent, heptane, which containsa trialkyl aluminium and a titaniumcompound. The product is amixture of isotactic and atacticpolypropene, the former being themajor constituent. Polypropene isused as a thermoplastic mouldingmaterial.
In PP production, propylene monomer is polymerized to
make the homopolymer by using a Ziegler–Natta type coordination
catalyst. This catalyst results from the reaction and
interaction of a transition metal compound and an organometallic
compound, usually an alkylaluminum compound.
Halide atoms are involved in most such catalyst systems .
Polypropylene can be made by solution, slurry (or solvent),
bulk (or liquid propylene), or gas-phase polymerization, or a
combination of these processes . The most widely used is
the slurry process; however, the current trend is toward the
gas-phase process. In the solution, slurry, and bulk processes,
the catalyst system is mixed with propylene and a hydrocarbon
diluent (usually hexane, heptane, or liquid propylene) in a
reactor. After polymerization, the reaction mixture enters a
flash tank where unreacted propylene is removed and
recycled. Propylene–ethylene copolymers [9010-79-1] can
be manufactured when ethylene is fed along with propylene to
the polymerization reactor or by adding ethylene and propylene
to a postpolymerization reactor that contains PP. This
mixture may then be purified to remove lowmolecular weight
and atactic fractions and washed to remove catalyst residues.
The polypropylene resin is then dried and pelletized. During this time, additivesmay be incorporated in the gas-phase
process; no liquid diluent is used .
High pressure, free radical processes of the type used to prepare polyethylene
are not satisfactory when applied to propylene and other tX-olefins bearing a
hydrogen atom on the carbon atom adjacent to the double bond. This is
attributed to extensive transfer of this hydrogen to propagating centres (R .):
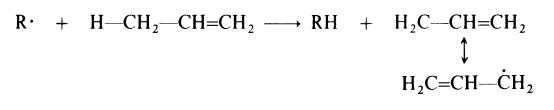
The resulting allyl radical is resonance stabilized and has a reduced tendency
to react with another monomer molecule.
Although the Phillips and Standard Oil processes can be used to prepare
polypropylene, the polymer yields tend to be low and it appears that these
processes have not been used for commercial production of polypropylene.
Until about 1980, polypropylene has been produced commercially only by
the use of Ziegler-Natta catalysts. Commonly a slurry process is used and is
carried out in much the same manner as described previously for the
preparation of polyethylene ). In the case of polypropylene, some atactic polymer is formed besides the required isotactic polymer;
but much of this atactic material is soluble in the diluent (commonly heptane)
so that the product isolated is largely isotactic polymer. Recently, there has
been a marked shift towards processes involving gas phase polymerization
and liquid phase polymerization. Few details of these newer processes have
been published. Gas phase processes resemble those described previously for
the preparation of polyethylene ) and swing plants are
now feasible. In liquid phase processes polymerization is conducted in liquid
propylene, typically at 2 MPa (20 atmospheres) and 55??C. Concurrently with
these developments, new catalyst systems have been introduced. These materials have very high activity and the reduced levels that are required make it
unnecessary to remove catalyst from the final polymer. Also, the new catalyst
systems lead to polypropylene with higher'proportions of isotactic polymer
and removal of atactic polymer is not necessary.
Tan to white odorless solid. Less dense than water and insoluble in water. Hence floats on water.
Polypropylene reacts with chlorine, fuming nitric acid and other strong oxidizing agents.
Polypropylene is similar in structure to polyethylene,but every other carbon atom has oneof its H2 atoms replaced by a CH2group.Although electrically similar to polyethylene,polypropylene can be made in thinner films, say 5μm as against about 25 μm for polyethylene.These films replace paper for impregnatedcapacitors, with reduced loss.
Moderately toxic by
ingestion and intraperitoneal routes.
Questionable carcinogen. When heated to
decomposition it emits acrid smoke and
irritating fumes. Used in injection molding for auto parts, in bottle caps, and in
container closures.
No data on the carcinogenicity
and mutagenicity of propylene are available for evaluation
by the working group.