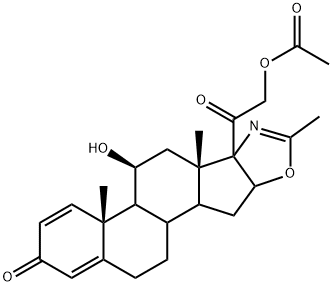
Deflazacort (trade name Emflaza among others) is a glucocorticoid used as an anti-inflammatory and immunosuppressant. It belongs to a group of medicines called corticosteroids. It is sometimes referred to simply as an oral steroid.
Deflazacort is an inactive prodrug which is metabolized rapidly to the active drug 21-desacetyldeflazacort.It is used to treat a wide variety of conditions. Some examples include autoimmune diseases (for example, systemic lupus erythematosus (SLE), autoimmune hepatitis, sarcoidosis), joint and muscle diseases (for example, rheumatoid arthritis), and allergies and asthma.
Cardiff can be specially designed for the third generation of corticosteroids, anti-inflammatory, anti-allergy, and it increased gluconeogenesis and so on. It acts as prednisolone 10~20 times of the dragon, 40 times as hydrocortisone. This product has no mutagenic effect. This product, labeled with C14 study, shows that the product of the gastrointestinal tract rapidly absorbed, reaching the peak blood concentration after 1~2h, forming the active substance by hydrolysis, and the latter further metabolic processes is similar with other corticosteroids, as well as the active metabolite T1/2 is 2 h and the rats are mainly distributed in the kidney and hematopoietic system and other organizations.
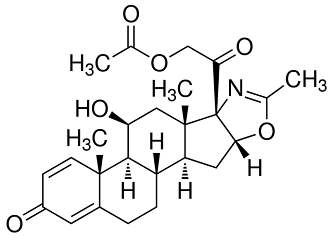
Figure 1 is a deflazacort formula.
Acetone-hexane, m.p. 255-256.5 ℃. [Α] D + 62.3 ° (C = 0.5, chloroform). UV absorption maximum (methanol): 241~242nm (E1cm1% 352.5). Acute toxicity LD50 in mice (mg/kg): 5200 oral; 1610 subcutaneous injection. Rats Acute toxicity LD50 (mg/kg): 109 subcutaneously. Dog Acute toxicity LD50 (mg/kg):> 4000 Oral; 50 subcutaneous injection.
The third generation of corticosteroids, anti-inflammatory, anti-allergy, increased gluconeogenesis and so on. For the use of the primary and secondary adrenocortical insufficiency, rheumatism, collagen diseases, skin diseases, allergic diseases, eye diseases, and fulminant disseminated tuberculosis, hematopoietic system disorders, ulcerative colitis, idiopathic nephrotic syndrome, and other hematopoietic malignancies.
Deflazacort carries the risks common to all corticosteroids, including immune suppression, decreased bone density, and endocrine insufficiency. In clinical trials, the most common side effects (>10% above placebo) were Cushing's-like appearance, weight gain, and
increased appetite.
Long-term medication can affect the normal growth of children, induced peptic ulcer perforation, which can cause euphoria, depression, insomnia and other psychiatric symptoms. Sudden withdrawal after long-term use can cause secondary adrenal insufficiency withdrawal reactions, which should gradually decrease until disabled.
- Like other glucocorticoids, it is general contraindicated in systemic infections.
- Used with caution in diverticulitis, recent gastrointestinal surgery, renal failure, hypertension, diabetes, osteoporosis, myasthenia gravis embolism. Pregnancy and lactation women should used with caution.
- Cardiff may have unique row potassium, and it should pay more attention when combined with diuretics.
- When combined with the drug having enzymatic action (rifampicin, phenobarbital, etc.), the corticosteroids should be properly increment.
- Erythromycin, estrogen can inhibit the activity of this product and the metabolism, when combined with appropriate reductions.
Compound 10g (1) was dissolved in 350ml methanol, and then added 8.3g of semicarbazide hydrochloride and 5.75ml of pyridine in 50ml of water, after the reaction. We can obtain compound 11g (Ⅱ). Compound 9g (Ⅱ) was dissolved 230ml 95% ethanol, the solution was added 3.6g of potassium carbonate and 2.34g of sodium borohydride in 36ml of water, which was refluxed for 30min and then added 2.34g of sodium borohydride. After the reaction, we can obtain compound 8.5g (Ⅲ). Compound 7.7g (Ⅲ) was dissolved in 154ml 10% hydrochloric acid in methanol, refluxed for 1h, to give Compound 6g (Ⅳ). Compound 12.5g (Ⅳ) was dissolved in 500ml of anhydrous toluene and 90ml of cyclohexanone. It was added 6.24g triisopropyl aluminum, 9g obtained by reacting the compound (V). 6.5g compound (V) was dissolved in 123ml dry dioxane, and 25% hydrogen bromide in acetic acid solution is heated, and then added 5.5g of bromine in dioxane; the reaction solution was poured into the water, collected by filtration after the crystals were carefully dried, redissolved in dimethylformamide. It was added 7.5g of lithium bromide and lithium carbonate (1: 2) mixture. After heating the reaction, we can obtain the compound 5.1g (ⅵ). 2g compound (Ⅵ) was dissolved in tetrahydrofuran-methanol (1: 1), added to 3.9g calcium oxide and 0.1g of azobisisobutyronitrile. It was added 2g of iodine in tetrahydrofuran-methanol mixture; After the reaction was filtered off precipitate , which was dissolved in acetone, it was then added triethylamine 20ml, 20ml and 12ml acetone, acetic acid, atter the reaction, we can obtain 1.6g deflazacort.
https://en.wikipedia.org/wiki/Deflazacort
http://patient.info/medicine/deflazacort-tablets-calcort
Deflazacort is an antiinflammatory
treatment of rheumatoid arthritis. Its antirheumatic potency i s similar to that of prednisone, while its hyperglycernic and calcium depleting effects are reportedly less.
Off-White to Pale Yellow Solid
A systemic corticosteroid. A derivative of prednisolone. Used for rheumatoid arthritis and lupus
Deflazacort-d3, is the labeled analogue of Deflazacort (D228975), a systemic corticosteroid, and a derivative of prednisolone. Used for rheumatoid arthritis and lupus.
ChEBI: Deflazacort is a corticosteroid hormone.
Deflazacort is an anti-inflammatory and immunosuppressant glucocorticoid.
Glucocorticoid:
Suppression of inflammatory and allergic disorders
Potentially hazardous interactions with other drugs
Aldesleukin: avoid concomitant use
Antibacterials: metabolism accelerated by rifamycins;
metabolism possibly inhibited by erythromycin;
concentration of isoniazid possibly reduced.
Anticoagulants: efficacy of coumarins and
phenindione may be altered.
Antiepileptics: metabolism accelerated by
carbamazepine, fosphenytoin, phenobarbital,
phenytoin and primidone
Antifungals: increased risk of hypokalaemia with
amphotericin - avoid; metabolism possibly inhibited
by itraconazole and ketoconazole
Antivirals: concentration possibly increased by
ritonavir.
Ciclosporin: rare reports of convulsions in patients
on ciclosporin and high-dose corticosteroids;
increased half-life of deflazacort.
Cobicistat: concentration increased by cobicistat -
avoid.
Diuretics: enhanced hypokalaemic effects of
acetazolamide, loop diuretics and thiazide diuretics.
Vaccines: high dose corticosteroids can impair
immune response to vaccines; avoid with live
vaccines.
Deflazacort is immediately converted by plasma
esterases to the pharmacologically active metabolite (D
21-OH). It is 40% protein-bound and has no affinity for
corticosteroid-binding-globulin (transcortin). Elimination
takes place primarily through the kidneys; 70% of the
administered dose is excreted in the urine. The remaining
30% is eliminated in the faeces. Metabolism of D 21-OH
is extensive; only 18% of urinary excretion represents
D 21-OH. The metabolite of D 21-OH, deflazacort
6-beta-OH, represents one third of the urinary
elimination.