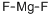Magnesium fluoride is a by-product of the manufacture of metallic beryllium and uranium. It is a fine white crystalline powder with low chemical reactivity.

Magnesium fluoride is used as flux in magnesium metallurgy and in the ceramics industry. Magnesium fluoride may be used for the extraction of aluminum from arc-furnace alloys with Fe, Si, Ti, and C. Optical windows of highly purified magnesium fluoride which transmit light from the vacuum ultraviolet (140 nm) into the infrared (7) are recommended for use as ultraviolet optical components for the use in space exploration. Magnesium fluoride is can be also used as an antireflection coating material having a good antireflection effect and a low refractive index.
In Mg metallurgy and in the ceramics industry, Magnesium fluoride is used as a flux. Single crystals of alkaline-earth fluorides, such as Magnesium fluoride, are suitable for optical applications because of their large domain of transparency from the ultraviolet to the middle infrared region. Infrared transparent windows may be prepared by hot-pressing Magnesium fluoride powder.
Ternary intercalation compounds of graphite with fluorine and Magnesium fluoride have been prepared; these compounds have high electrical conductivity and would therefore have an important potential as cathodes or new electroconductive materials. Finally, the eutectic NaF–MgF2 has been proposed in advanced latent-heat energy storage for solar power systems.
Magnesium fluoride is prepared by treating a magnesium salt solution with hydrofluoric acid or sodium fluoride:
MgSO4 + 2HF → MgF2 + 2H+ + SO42–
or by adding hydrofluoric acid to magnesium carbonate:
MgCO3 + 2HF → MgF2 + CO2 + H2O
[1] John R. Papcun, Fluorine Compounds, Inorganic, Magnesium, Kirk- Othmer Encyclopedia of Chemical Technology, 2000
[2] H. Tanaka, M. Kobayashi, T. Sakakibara, Method of producing magnesium fluoride coating, antireflection coating, and optical element, Patent, 2013
Magnesium fluoride (MgF2) is a white crystalline salt.
It is used in the electrolysis of aluminum ore to produce
metallic aluminum and also as a reflective coating on
various types of optical components. It is a tetragonal,
birefringent crystal with the TiO2 type of structure.
white to light beige powder
Magnesium Fluoride is a fine white crystalline powder with low chemical reactivity. This relative inertness makes possible some of its uses, eg, stable permanent films to alter light transmission properties of optical and electronic materials.
Magnesium fluoride (MgF2) is used to polarize corrective lenses of eyeglasses to reduce the
glare of sunlight by selecting the orientation of the light waves passing through the lenses.
MgF2 is also used to polarize windows, sunglasses, and similar optical items.
Suitable for vacuum deposition. Magnesium fluoride occurs in nature as the mineral, sellaite. It is used in
glass and ceramics. Single crystals are used for polarizing prisms and lenses.
In the ceramics and glass industry.Magnesium Fluoride is a durable crystal with low absorption, suitable for high-powered laser, space, and other UV applications. MgF2 is also naturally birefringent, making it an ideal material for use where this property can be exploited, such as retardation plates and polarizing elements, particularly in the wavelength range from 0.13-0.30 µm.
Magnesium fluoride is a colorless salt with the rutile structure. It is formed by reaction of magnesium oxide and HF or magnesium carbonate and NH4F·HF. It is also a by product from the manufacture of elements such as beryllium by reduction of the corresponding fluoride by magnesium metal.
Strong irritant. TLV: 2.5 mg(F)/m3.
Moderately toxic by
ingestion. When heated to decomposition it
emits toxic fumes of F-. See also
MAGNESIUM and FLUORIDES.