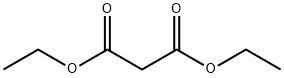
As an organic compound, diethyl malonate belongs to the diethyl ester of malonic acid, which is present naturally in guava fruits, melons, grapes, pineapples, blackberries and strawberries as a colorless liquid with an apple-like odor. It is a flavor ingredient commonly found in perfumes, artificial flavorings, alcoholic beverages, various wines and spirits due to its natural pleasant odor. It is also used as an essential intermediate in the syntheses of numerous pharmaceuticals, such as barbiturates, vitamins B1 and B6, non-steroidal anti-inflammatory agents. Besides, diethyl malonate is also involved in organic synthesis of other compounds, such as alpha-aryl malonates, mono-substituted and di-substituted acetic acid. And it can react with benzaldehyde for the production of diethyl benzylidenemalonate in Knoevenagel condensation reaction.
Diethyl malonate is the diethyl ester of malonic acid. It naturally occuring in grapes and strawberries, is widely used in the manufacture of pharmaceuticals, antioxidants, and dyes.
Diethyl malonate is used in organic synthesis for the preparation of alpha-aryl malonates, mono-substituted and di-substituted acetic acid, barbiturates and artificial flavorings. It is also involved in the synthesis of pharmaceuticals like chloroquine, butazolidin and barbital. It acts as intermediate in the synthesis of vitamin B1, vitamin B6, non-steroidal anti-inflammatory agents agrochemicals and perfumes. In Knoevenagel condensation reaction, it reacts with benzaldehyde to get diethyl benzylidenemalonate.
https://en.wikipedia.org/wiki/Diethyl_malonate
https://pubchem.ncbi.nlm.nih.gov/compound/7761#section=Safety-and-Hazards
https://www.alfa.com/zh-cn/catalog/A15468/
http://www.hmdb.ca/metabolites/HMDB29573
http://www.chemicalland21.com/industrialchem/organic/DIETHYL%20MALONATE.htm
Diethyl malonate is a diester derivative of malonic acid, a dicarboxylic acid with two carboxyl groups (-COO-) separated by one methylene group (-CH2-). Diethyl malonate is formed by the replacement of the hydroxyl groups (-OH) of malonic acid with ethoxy groups (-OCH2CH3). The hydrogen atoms on the methylene carbon between the two carboxyl groups make this compound acidic. Because of its unique structure, diethyl malonate is reactive and functions as a reagent for organic synthesis and to make products such as barbiturates, pigments, and agrochemicals. Volatile esters are known to have fruity scents and are often used as fragrances and flavorings. Diethyl malonate is a volatile diester that occurs naturally in fruits such as grapes, strawberries, guava, melon, pineapple, and blackberries.
Diethyl malonate has a faint, pleasant, aromatic odor.
Reported found in pineapple, bilberry, Cape gooseberry, cognac, malt whiskey, apple brandy, grape brandy,
port, cider, sherry and red, white, strawberry and bilberry wines.
Diethyl Malonate occurs naturally in grapes and strawberries. It is used in the preparation of barbiturates, artificial flavourings, vitamin B1, and vitamin B6 as well as in perfumes.
Diethyl malonate is used in organic synthesis for the preparation of alpha-aryl malonates, mono-substituted and di-substituted acetic acid, barbiturates and artificial flavorings. It is also involved in the synthesis of pharmaceuticals like chloroquine, butazolidin and barbital. It acts as intermediate in the synthesis of vitamin B1, vitamin B6, non-steroidal anti-inflammatory agents agrochemicals and perfumes. In Knoevenagel condensation reaction, it reacts with benzaldehyde to get diethyl benzylidenemalonate.
manufacture of barbiturates.
Reacting chloroacetic acid to cyanoacetic acid using sodium cyanide and subsequent saponification; malonic acid is
finally esterified by azeotropic distillation with ethanol in benzene
ChEBI: Ethyl malonate is a dicarboxylic acid.
Taste characteristics at 50 ppm: sweet and fruity with apple and pineapple nuances.
Diethyl malonate is diethyl ester of malonic acid. Acylation of diethyl malonate using magnesium chloride and triethylamine is reported. K2CO3-catalyzed 1,4-addition reaction of diethyl malonate with various substituted 1,2-allenic ketones yields polyfunctionalized β,γ-unsaturated enones.
Mildly toxic by
ingestion. A skin irritant. Combustible liquid
when exposed to heat or flame; can react
with oxidizing materials. To fight fire, use
water to blanket fire, foam, CO2, dry
chemical. When heated to decomposition it
emits acrid smoke and irritating fumes. See
also ESTERS.
When the ester was fed to chicks at a level of 5% in the diet, 32% of the energy from diethyl malonate was available (Yoshida et al. 1970). Hydrolysis of diethyl malonate would produce ethanol and malonic acid, which is a relatively strong acid and acts as an inhibitor of enzymes, including succinic dehydrogenase (Fassett, 1963). Malonic acid injected into rats or rabbits is excreted largely unchanged, but also causes increased excretion of citric and a-ketoglutaric acids (Krebs, Salvin & Johnson, 1938). Some malonate may be metabolized through the tricarboxylic acid cycle, with decarboxylation to acetate followed by transformation to succinate, which has been detected in rat urine (Lee & Lifson, 1951). Diethyl malonate was hydrolysed by adipose-tissue lipase (Lynn & Perryman, 1960) and to the monoester by α-chymotrypsin (Cohen & Crossely, 1964). It was oxidized in 110 min to the extent of 34% by the homogenized mycelium of urethane-grown Streptomyces nitrifica (Schatz, Trelawny, Schatz & Mohan, 1957).
If too impure (IR, NMR) the ester (250g) is heated on a steam bath for 36hours with absolute EtOH (125mL) and conc H2SO4 (75mL), then fractionally distilled under reduced pressure. Otherwise fractionally distil it under reduced pressure and collect the steady boiling middle fraction. [Beilstein 2 IV 1881.]