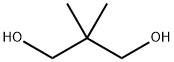
The chemical formula for Neopentyl glycol (NPG) is C5H12O2, and it is a propane-1,3-diol carrying two methyl groups at position 2.Neopentyl glycol is soluble in water, benzene, chloroform, and very soluble in ethanol and diethyl ether. Neopentylglycol (NPG) is a unique polyalcohol offering superior performance advantages in many end-use applications due to its high chemical and thermal stability. It is a unique diol offering superior performance advantages in many end-use applications. These advantages are derived from its chemical structure. The location of the hydroxyl groups on primary carbon atoms allows rapid esterification. Additionally, the two methyl groups, instead of the usual two hydrogen atoms, on the alpha carbon atom are responsible for the high chemical and thermal stability of Neopentylglycol and its derivatives.
Neopentyl glycol is used in the synthesis of polyesters, paints, lubricants, and plasticizers. When used in the manufacture of polyesters, it enhances the stability of the product towards heat, light, and water. By esterification reaction with fatty or carboxylic acids, synthetic lubricating esters with reduced potential for oxidation or hydrolysis, compared to natural esters, can be produced. NPG is used primarily in base resins for coatings. In powder coating formulations, Neopentylglycol offers the additional advantage of providing small differences between the glass transition temperature and melting point. Important areas of application include general metal, appliance, metal furniture, automotive and machinery coatings. Important uses are also found in hydraulic fluids, synthetic lubricant oils, greases, metalworking fluids and aircraft engine lubricants. Other outlets include textiles, pharmaceuticals, pesticides, plasticizers and petroleum.
High quality NPG is shipped as flake, molten and slurry. The high-quality NPG is the component in polyester resins for industrial coatings and fiberglass-reinforced plastics applications, and most polyester resin formulations contain NPG as the sole glycol component, or it is used in conjunction with a modifying glycol to achieve desired properties. NPG is also used in polyester polyols for polyurethane coatings for the automotive, industrial maintenance, transportation, and aerospace markets.
2,2-Dimethyl-1,3-propanediol may be used in the synthesis of:
- 2,2-dimethyl-1,3-propanediol cyclic phosphorochloridate
- 2,2-dimethyl-1,3-propanediol cyclic phenylphosphonate
- [1′,3′-(2′,2′-dimethylpropylene)]-2-iodo-3-octyl-5-thienylboronate
- cyclic carbonate
- 2,2-dimethyl-1,3-propanediol bis(cyclic-2,2-dimethyltrimethylene phosphite)
In the manufacture of plasticizers, polyesters, as modifier of alkyd resins.
NPG Glycol is used in the synthesis of tetraphenylporphyrins. Also used in the synthesis of Bryostatin 2, a protein kinase modulator.
3-hydroxy-2,2-dimethylpropanal) could be reduced to neopentyl glyco. Hydroxypivaldehyde can be reduced either by a crossed Cannizzaro reaction with equimolar amounts of formaldehyde and a base, or by catalytic hydrogenation. If inorganic bases such as potassium carbonate or sodium hydroxide are used as catalysts, satisfactory yields (ca. 80 %) of neopentyl glycol can be obtained only with an excess of isobutyraldehyde. Tertiary amines are also used as catalysts for the aldol addition. Catalytic transfer hydrogena�tion of the aldol intermediate is also possible using formate salts as hydrogen source. After fractional distillation a very good yield of high-purity neopentyl glycol is obtained.
ChEBI: Neopentyl glycol is a propane-1,3-diol carrying two methyl groups at position 2.
White crystalline solid. Melting point 130°C.
2,2-Dimethyl-1,3-propanediol may generate toxic gases in combination of with alkali metals, nitrides, and strong reducing agents. Reacts with inorganic acids and carboxylic acids to form esters plus water. Converted to aldehydes or acids by oxidizing agents. May initiate the polymerization of isocyanates and epoxides.
In addition to the reactions typical of primary alcohols, such as ester, ether, and carbamate (urethane) formation, 1,3-diols give six-membered cyclic derivatives with carbonyl compounds, carbonates, phosphites, sulfifites, and borates.
May be harmful by ingestion or skin absorption. Causes eye and skin irritation. Material is irritating to mucous membrane and upper respiratory tract. INHALATION: Call for medical aid. Remove to fresh air. If breathing has stopped, give artificial respiration. If breathing is difficult, give oxygen.
Special Hazards of Combustion Products: Emits toxic fumes under fire condition.
Flammability and Explosibility
Non flammable
Neopentyl glycol is soluble in water, alcohols, and ketones, moderately soluble in hot
aromatic solvents, such as benzene and toluene,
and relatively insoluble in aliphatic and cycloaliphatic solvents.
During storage, higher temperatures and
stacking pressures can lead to caking of the lower
stack layers. As a hygroscopic material, 2,2-Dimethyl-1,3-propanediol must be stored dry. The product is also
sold as 90 % aqueous solution, which should be
stored at 55 – 60 ℃ to avoid precipitation, and as
a 100 % melt.
Crystallise the diol from *benzene or acetone/water (1:1). [Beilstein 1 IV 2551.]