Sodium gluconate is the organic sodium salt of gluconic acid. Sodium gluconate is a chelator that forms stable complexes with various ions and ultimately prevents these ions from engaging in chemical reactions. Gluconates are naturally occurring substances that freely dissociate to the gluconate anion and its respective cations. Being fully biodegradable and non-toxic, it represents an environment friendly alternative to the common chelating agents used in cosmetics such as EDTA. In addition to this, sodium gluconate has a low acute toxicity to aquatic organisms.
Sodium gluconate is a white to tan, granular to fine, practically odourless crystalline powder. It is very soluble in water, sparingly soluble in alcohol and insoluble in ether.
Sodium gluconate is used as a natural preservative. It prevents the growth of microbes in our products to keep them safe for our consumers. It also works as a skin-conditioning agent and a chelating agent which helps cleansing products to foam better in hard water. You can often find sodium gluconate in soap, sunscreen, shampoo, toothpaste, hair products, makeup, and a variety of other personal care products.
Sodium gluconate has been used as a component of recording buffer used in two-electrode voltage-clamp (TEVC) recording in Xenopus laevis oocytes. It has also been used as a control for sodium.
Sodium gluconate is manufactured by the fermentation of carbohydrate containing the raw material glucose syrup derived from maize. After a crystallisation step, sodium gluconate is separated from the mother liquor by centrifugation, the crystals are dried and then sieved to guarantee the desired granulation. Based on the production process as well as the raw materials used, sodium gluconate is not synthetic natural.
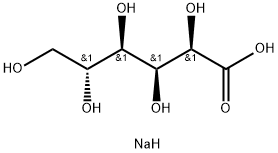
ChEBI: Sodium gluconate is an organic sodium salt having D-gluconate as the counterion. It has a role as a chelator. It contains a D-gluconate.
Ingestion of sodium gluconate is known to stimulate the production of intestinal butyrate. It is widely used in food, pharmaceutical paper and textile industry. It acts as a chelating agent. Sodium gluconate serves as a detergent in bottle washing formulation.
Low toxicity by intravenous route. When heated to decomposition it emits acrid smoke and irritating fumes
The calcium gluconate is added into the reaction kettle. Add sulfuric acid aqueous solution while stirring. After mixing for one hour, let it stand for a while and then get it filtered. The filter residue is CaSO4 and gets it removed. The filtrate is added into the neutralization kettle, and a proper amount of Na2CO3 aqueous solution is added to neutralize it. Finally get sodium gluconate through concentration, filtration and drying.
Crystallise it from a small volume of H2O (solubility is 59g/100mL at 25o), or dissolve it in H2O and add EtOH since it is sparingly soluble in EtOH. It is insoluble in Et2O. It forms a Cu complex in alkaline solution and a complex with Fe in neutral solution. [cf p 639, Sawyer & Bagger J Am Chem Soc 81 5302 1959, Beilstein 3 I 188.]
In Europe, sodium gluconate is listed as a generally permitted food additive (E576) and may be added to all foodstuffs, following the "quantum satis" principle, as long as no special regulation restricts the use.
The US Food and Drug Administration (FDA) assigned sodium gluconate the “generally recognised as safe” (GRAS) status and permitted its use in food without limitation other than current good manufacturing practice.
Sodium gluconate is exempted from the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) by means of Commission Regulation (EC) No. 987/2008 of 8 October, 2008 (amending Annex IV). As a consequence thereof, there is no need to register sodium gluconate.