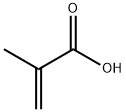
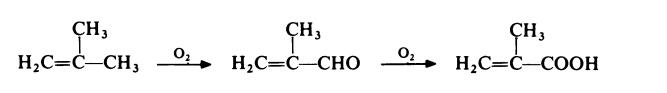Methacrylic acid, abbreviated MAA, is an organic compound. This colourless, viscous liquid is a carboxylic acid with an acrid unpleasant odor. It is soluble in warm water and miscible with most organic solvents. Methacrylic acid is produced industrially on a large scale as a precursor to its esters, especially methyl methacrylate (MMA) and poly(methyl methacrylate) (PMMA). The methacrylates have numerous uses, most notably in the manufacture of polymers with trade names such as Lucite and Plexiglas. MAA occurs naturally in small amounts in the oil of Roman chamomile.
Methacrylic acid is is a colorless, moderately volatile, corrosive liquid with a strongly acrid odor. It was first prepared in 1865 from ethyl methacrylate, in turn obtained by dehydration of ethyl α-hydroxyisobutyrate.
Methacrylic acid is used in the manufacture of methacrylate resins and plastics. It is used as Monomer for large-volume resins and polymers, organic synthesis. Many of the polymers are based on esters of the acid, as the methyl, butyl, or isobutyl esters. Methacrylic acid and methacrylate esters are used to prepare a wide range of polymers [→ Polyacrylamides and Poly(Acrylic Acids), → Polymethacrylates]. Poly(methyl methacrylate) is the primary polymer in this category, and it provides water-clear, tough plastics that are used in sheet form in glazing, signs, displays, and lighting panels.
The most common route for the preparation of methacrylic acid is from
acetone as follows:
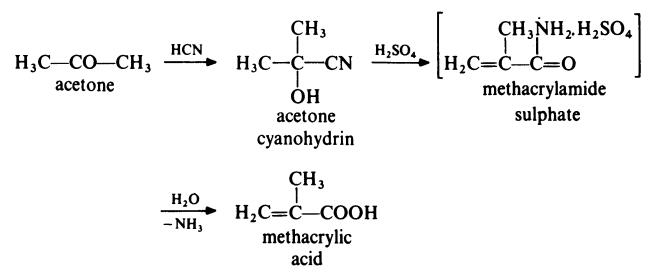
In a typical process, acetone is treated with hydrogen cyanide at 140??C in the
presence of ammonia as catalyst. The acetone cyanohydrin produced is
treated with concentrated sulphuric acid at 100??C to form methacrylamide
sulphate. This intermediate is not isolated but is directly converted to
methacrylic acid by treatment with water at about 90??C.
A competitive route now in commercial operation involves the two stage
oxidation of isobutene with air. The reaction proceeds via methacrolein:
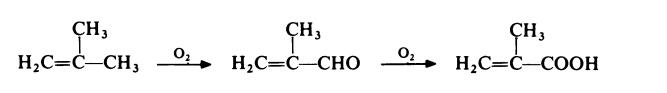
The most common approach to methacrylic acid synthesis is the hydrolysis of methacrylamide sulfate, obtained from acetone cyanohydrin. Methyl methacrylate may be prepared directly in a similar way by adding methanol in the final reaction step.
In the manufacture of methacrylic acid, methacrylamide sulfate is reacted with water under conditions similar to those used for formation of the ester. The reactor effluent separates into two phases. The upper organic layer is distilled to provide pure methacrylic acid. The lower layer is steam stripped to recover dilute aqueous methacrylic acid, which is recycled to the hydrolysis reactor. The waste acid stream is treated as in the manufacture of the ester.
ChEBI: Methacrylic acid is an alpha,beta-unsaturated monocarboxylic acid that is acrylic acid in which the hydrogen at position 2 is substituted by a methyl group. It is functionally related to an acrylic acid. It is a conjugate acid of a methacrylate.
Methacrylic acid appears as a clear colorless liquid (or low-melting solid) with a pungent odor. Corrosive to metals and tissue. Flash point 170°F. Melting point 61°F. May polymerize exothermically if heated or contaminated. If the polymerization takes place inside a container, the container may rupture violently. Less dense than water. Vapors heavier than air. Used to make plastics.
Methacrylic acid reacts with strong oxidizing agents. Presents a storage hazard: violent exothermic polymerizations leading to explosion can occur spontaneously, particularly at low inhibitor or stabilizer concentrations [Anon., CISHC Chem. Safety Summ., 1979, 50, p. 34; Bond, J., Loss Prev. Bull., 1991, 101, p. 1].
Methacrylic acid is a highly corrosive liquid.Contact with eyes can result in blindness.Skin contact may produce burns. No inhalation toxicity was observed in rats. Exposureto its vapors may produce skin and eye irritation, which can be mild to moderate. Adermal LD50 value in rabbits is 500 mg/kg.
Combustible liquid; flash point (open cup)
76°C (170°F); vapor pressure <0.1 torr at
20°C (68°F). Fire-extinguishing agent: water
spray, “alcohol” foam, dry chemical, or CO2;
use a water spray to dilute and flush the spill
and to disperse the vapors.
Methacrylic acid polymerizes readily. The
reaction is exothermic. The rate of reaction
accelerates on heating, which may result
in violent rupture of closed containers. The
polymerization may be inhibited with a trace
quantity of hydroquinone and hydroquinone
monomethyl ether (Aldrich 2006). The acid
may be stored safely below its melting point.
Poison by
intraperitoneal route. Moderately toxic by
ingestion and skin contact. Corrosive to
skin, eyes, and mucous membranes.
Mutation data reported. Flammable when
exposed to heat, flame, or oxidizers. A
storage hazard; exothermic polymerization
may occur spontaneously. To fight fire, use
alcohol foam, spray, mist, dry chemical.
When heated to decomposition it emits
acrid smoke and irritating fumes.
Methacrylic acid is used in preparation of methacrylates and carboxylated polymers; in the
production of the material or its alkyl esters, as monomers
or comonomers for synthetic resins for the production of
plastic sheets, moldings, and fibers.
If this chemical gets into the eyes, remove any contact lenses at once and irrigate immediately for at least 15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart action has stopped. Transfer promptly to a medical facility. When this chemical has been swallowed, get medical attention. If victim is conscious, administer water or milk. Do not induce vomiting. Medical observation is recommended for 2448 h after breathing overexposure, as pulmonary edema may be delayed. As first aid for pulmonary edema, a doctor or authorized paramedic may consider administering a corticosteroid spray.
Methacrylic acid was considered
by the IARC Working Groups, but monographs were not
prepared because adequate data on its carcinogenicity were
not available. The IUCLID database reports a
dermal application study (dose unspecified) of mice treated
three times per week for 4 months and then observed for their
lifetimes. No excess dermal tumors were observed.
(1) Color Code—White: Corrosive or Contact Hazard; Store separately in a corrosion-resistant location. (2) Color Code—Blue: Health Hazard/Poison: Store in a secure poison location. (3) Color Code—Yellow Stripe (strong reducing agent): Reactivity Hazard; Store separately in a area isolated from flammables, combustibles, or other yellow coded materials. Prior to working with this chemical you should be trained on its proper handling and storage. Before entering confined space where this chemical may be present, check to make sure that an explosive concentration does not exist. Store in tightly closed containers in a cool, well-ventilated area away from oxidizers (such as perchlorates, peroxides, permanganates, chlorates, and nitrates). Methacrylic acid should be stored at temperatures below 15℃. Sources of ignition, such as smoking and open flames are prohibited where methacrylic acid is handled, used, or stored. Wherever methacrylic acid is used, handled, manufactured, or stored, use explosion-proof electrical equipment and fittings.
UN2531 Methacrylic acid, stabilized, Hazard
class: 8; Labels: 8-Corrosive material.
Aqueous methacrylic acid (90%) is saturated with NaCl (to remove the bulk of the water), then the organic phase is dried with CaCl2 and distilled under vacuum. Polymerisation inhibitors should be added to the distillate and include 0.25% p-methoxyphenol, 0.1% hydroquinone, or 0.05% N,N'-diphenyl-p-phenylenediamine. [Beilstein 2 IV 1518.]
Vapor may form explosive mixture
with air. A reducing agent; incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or
explosions. Aqueous solution is strongly acidic: incompatible
with strong acids; caustics, ammonia, amines, isocyanates,
alkylene oxides; epichlorohydrin. Will polymerize readily from
heating above 59�F/15�C, or due to the presence of light, oxidizers (e.g., peroxides); or in the presence of traces of hydrochloric acid, with fire or explosion hazard. Attacks metals. Note:
Typically contains 100 ppm of monomethyl ether hydroquinone (150-76-5) as an inhibitor to prevent polymerization
Dissolve or mix the material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal,
state, and local environmental regulations must be
observed.