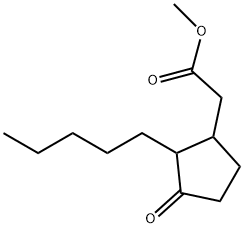
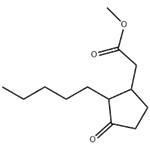
Methyl dihydrojasmonate has a powerful sweet-floral, jasminelike, somewhat fruity odor. This is the odoriferous component in
jasmine oil (Jasminum gradiflorum L.). May be prepared by condensation of 2-pentyl-2-cyclopenten-l-one with ethyl malonate,
followed by hydrolysis, decarboxylation, and methylation.
Methyl dihydrojasmonate has a powerful sweet-floral, jasmine-like, somewhat fruity odor. This compound is the odor�iferous component of jasmine oil (Jasminum gradiflorum L.)
Methyl dihydrojasmonate is a jasmine fragrance
that is closely related to methyl jasmonate, which occurs in jasmine oil. Methyl
dihydrojasmonate has been identified in tea. It is a liquid with a typical fruity,
jasmine-like blossom odor.
Methyl dihydrojasmonate is prepared by Michael addition of malonic acid
esters to 2-pentyl-2-cyclopenten-l-one, followed by hydrolysis and decarboxylation
of the resulting (2-pentyl-3-oxocyclopentyl)malonate, and esterification of
the (2-pentyl-3-oxocyclopentyl)acetic acid [304]. 2-Pentyl-2-cyclopenten-1-one
is prepared by an aldol condensation between cyclopentanone and valeraldehyde
and subsequent isomerization of the resulting 2-
pentylidenecyclopentanone or by palladium catalyzed decarboxylation
of allyl 2-oxo-1-pentylcyclopentanecarboxylates.
Dealkoxycarbonylation of the malonate can also be accomplished directly with
water at elevated temperature.
Methyl dihydrojasmonate of the aforementioned quality consists of a 9 : 1
equilibrium mixture of the trans- and cis-isomers. However, methyl cisdihydrojasmonate
is the much more intensive isomer, with a threshold about 20
times lower than that of the trans-isomer. Therefore, methyl dihydrojasmonate
qualities with enriched portions of the cis-isomer are also marketed.
These “high-cis” products are colorless liquids with extremely powerful jasmine
character.Thedifferent commercial qualities may contain different amounts of the
cis-isomer.
High-cis methyl dihydrojasmonate is a valuable material in fine fragrances but
suffers from stability problems due to its tendency to isomerize into the equilibrium
mixture and, therefore, has only limited usage in other perfumery applications.
High-cis methyl dihydrojasmonate can be produced from the equilibrium mixture
by special distillation techniques in which isomerization is effected by the
action of sodium carbonate.
A high proportion of cis methyl dihydrojasmonate can also be obtained
by hydrogenation of methyl dehydrodihydrojasmonate, which is accessible
from 1(2-furyl)-hexanol via rearrangement, isomerization, etherification, and
condensation with dimethyl malonate.
For other stereoselective synthetic approaches, see review.
Of all possible isomers, the (+)-(1R)-cis-isomer possesses the most characteristic
and intensive jasmine odor.Therefore, an industrially feasible process for the
production of a methyl dihydrojasmonate with a high portion of this isomer has
been developed. The process comprises the catalytic hydrogenation of the corresponding
cyclopenteneacetic acid in the presence of a ruthenium(II) complexwith
chiral ligands and subsequent esterification.
Methyl dihydrojasmonate is used in perfumery for blossom fragrances, particularly in jasmine types.
Reported found in jasmine oil (Jasminum gradiflorum L.) and black tea
Methyl (3-Oxo-2-pentylcyclopentyl)acetate is used in the preparation of hydrogels as delivery systems for the slow release of bioactive carbonyl derivatives.
By condensation of 2-pentyl-2-cyclopenten-1-one with ethyl malonate, followed by hydrolysis, decarboxylation and
methylation
Taste characteristics at 20 ppm: sweet, floral, citrus, fruity and berry with tutti-frutti undernotes.
Methyl dihydrojasmonate is a fragrance ingredient mainly used in the perfumes, fragrance formulations, personal care and cosmetic products. It occurs naturally in pine honey and rhododendron honey.
Flammability and Explosibility
Non flammable
Claigeon®, Cepionate®(Nippon Zeon),Hedione®,Hedione®HC
(Firmenich), Kharismal® (IFF).