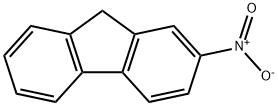
2-Nitrofluorene is a genotoxic compound and also a biomaker that affects the DNA of living organisms. An environmental pollutant.
ChEBI: A nitroarene that is fluorene substituted by a nitro group at position 2.
Aromatic nitro compounds, such as 2-Nitrofluorene, range from slight to strong oxidizing agents. If mixed with reducing agents, including hydrides, sulfides and nitrides, they may begin a vigorous reaction that culminates in a detonation. The aromatic nitro compounds may explode in the presence of a base such as sodium hydroxide or potassium hydroxide even in the presence of water or organic solvents. The explosive tendencies of aromatic nitro compounds are increased by the presence of multiple nitro groups. Stable under normal conditions. Protect from bases.
ACUTE/CHRONIC HAZARDS: Toxic. Hazardous decomposition products. Carcinogen.
Confirmed carcinogen
with experimental carcinogenic and
tumorigenic data. Moderately toxic by
intraperitoneal route. Human mutation data
reported. When heated to decomposition it
emits toxic fumes of NOx. See also NITRO
COMPOUNDS OF AROMATIC
HYDROCARBONS.
Lung microsomal metabolism of 2-nitrofluorene (NF)
increases in parallel with the accumulation of P-450b
homologous mRNA and microsomal cytochrome P-
450b protein concentration. The formation of the major
metabolite, and potent mutagen, 9-hydroxy-2-
nitrofluorene (9-OHNF) is significantly inhibited by the
addition of polyclonal anti-P-450b-IgG, and by the
addition of the inhibitor, proadifen, to incubations with
lung microsomal protein.
Crystallise 2-nitrofluorene from aqueous acetic acid or Me2CO (m 158-158.5o, also 160-160.5o). [Beilstein 5 H 628, 5 III 1948.]