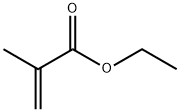
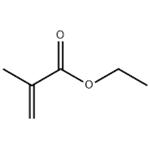
colourless liquid with an unpleasant odour
Ethyl methacrylate is a clear, colorless, and highly flammable
liquid that polymerizes but at a slower rate than that of
the parent acrylate.
Ethyl methacrylate can be manufactured via a reaction of
methacrylic acid or methyl acrylate with ethanol. It is used primarily for manufacturing polymers and as a component of
acrylic polymers for surface coatings and as a structural
monomer for some artificial fingernail formulations.
Ethyl methacrylate is used in dental protheses or in
photobonded sculptured nails.
Polymers, chemical intermediates.
Ethyl methacrylate can be manufactured via a reaction of
methacrylic acid or methyl acrylate with ethanol.
A colorless moderately toxic liquid with an acrid odor. Flash point of 70°F. Boiling point 278°F. Vapors irritate the eyes and respiratory system. Less dense than water. Not soluble in water. Used to make polymers and other chemicals.
Highly flammable. A very dangerous fire and explosion hazard. Not soluble in water.
May polymerize if heated for prolonged periods or accidentally contaminated. If polymerization takes place inside a container, the container may violently rupture. Can react with oxidizing materials. When heated to decomposition Ethyl methacrylate emits irritating fumes and acrid smoke [Sax, 9th ed., 1996, p. 1576].
Flammable, dangerous fire and explosion
hazard. An irritant.
Inhalation may cause irritation of the mucous membrane. Ingestion causes irritation of mouth and stomach. Contact with liquid irritates eyes and skin.
Behavior in Fire: Sealed containers may rupture explosively if hot. Heat can cause a violent polymerization reaction with rapid release of energy. Vapors are heavier than air and can travel to a source of ignition and flash back.
Flammability and Explosibility
Non flammable
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: If proper concentration of inhibitor is not present or when material is hot, a violent polymerization reaction may occur; Inhibitor of Polymerization: Oxygen in the air inhibits polymerization.
Moderately toxic by
ingestion and intraperitoneal routes. Mildly
toxic by inhalation. Experimental
teratogenic and reproductive effects. A skin
irritant. A very dangerous fire and explosion
hazard when exposed to heat, sparks, or
flame; can react with oxidzing materials. To
fight fire, use CO2, dry chemical. When
heated to decomposition it emits acrid
smoke and irritating fumes.
Not listed by ACGIH, California
Proposition 65, IARC, NTP, or OSHA.
Wash the ester successively with 5% aqueous NaNO2, 5% NaHSO3, 5% NaOH, then water. Dry it over MgSO4, add 0.2% (w/w) of phenyl-.-naphthylamine, and distil it through a short Vigreux column (p 11) [Schultz J Am Chem Soc 80 1854 1958]. [Beilstein 2 IV 1523.]