Diethylstilbestrol (DES) is a synthetic estrogen receptor agonist that was prescribed to pregnant women in the late 1930s. It was banned in 1971 because of possible links to increased risk of breast cancer in mothers along with congenital abnormalities and increased risk of cancer in offspring. DES is structurally related to, and is as potent as, estradiol in most assays, with a longer half-life. It has a relative binding affinity to sex hormone binding globulin (SHBG) of 0.2 compared to estradiol which has a relative binding affinity of 100. A concentration of 20 μM DES, displaces 30 ± 13% of 3H-estradiol from SHBG in serum.
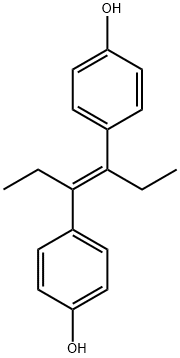
Diethylstilbestrol is an odorless, white crystalline powder, with a molecular weight of 268.36. Its cisisomer tends to revert to the trans-form. It is a nonsteroidal, synthetic stilbene derivative with estrogenic activity. recomended solvents are DMSO, DMF and ethanol, even in these solvents do not store in solution for any prolonged period of time.
Diethylstilbestrol is a synthetic nonsteroidal estrogen that was
formerly used in estrogenic hormone therapy (for menstrual
disorders, postpartum breast engorgement, postcoital contraceptive,
prevention of spontaneous abortion) and in chemotherapy
of various cancers, including postmenopausal breast
cancer and prostate cancer. It was also used in biomedical
research and veterinary medicine (growth promoter for cattle
and sheep; veterinary drug to treat estrogen deficiency disorders).
Diethylstilbestrol has been used:
to evaluate the estrogenic activity of diethylstilbestrol by quantitating the expression levels of endogenous estrogen-regulated marker genes
to evaluate the estrogenic, androgenic and toxicity responses in bioluminescent yeast bioreporter assay (BLYES)
to detect its effect on the proliferation and tyrosinase activity of melanocytes
ChEBI: Diethylstilbestrol is an olefinic compound that is trans-hex-3-ene in which the hydrogens at positions 3 and 4 have been replaced by p-hydroxyphenyl groups. It has a role as an antineoplastic agent, a carcinogenic agent, a xenoestrogen, an EC 3.6.3.10 (H(+)/K(+)-exchanging ATPase) inhibitor, an antifungal agent, an endocrine disruptor, an EC 1.1.1.146 (11beta-hydroxysteroid dehydrogenase) inhibitor, an autophagy inducer and a calcium channel blocker. It is a polyphenol and an olefinic compound.
Diethylstilbestrol is one of the older synthetic estrogens in use. It was used to treat prostate cancer but is now rarely used for this purpose because of its adverse effects,although it is occasionally used in postmenopausal women with breast cancer. It is taken orally in tablet form.
50 parts by weight of p-hydroxypropiophenone are dissolved in 200 parts by weight of a 12.5% solution of caustic soda and shaken with 350 parts by
weight of 3% sodium amalgam. The sodium salt of the pinacol thereby
precipitating is reacted with glacial acetic acid, whereby the free pinacol is
obtained (MP 205°C to 210°C, after purification 215°C to 217°C). The yield
amounts to 95% of the theoretical. The pinacol is suspended in ether and
gaseous hydrogen chloride introduced, whereby water separates and the
pinacolin formed is dissolved in the ether, from which it is obtained by
evaporation as a viscous oil (diacetateof MP 91°C). The yield is quantitative.
40 parts by weight of pinacolin are dissolved in ethyl alcohol and gradually
treated with 80 parts by weight of sodium under reflux. The solution is
decomposed with water and the pinacolin alcohol formed extracted from the
neutralized solution with ether. The pinacolin alcohol is a viscous oil which is
characterized by a dibenzoate of MP 172°C. The yield is 95% of the
theoretical.
A solution of 30 parts by weight of pinacolin alcohol in ether is saturated with
hydrogen chloride at room temperature and the ether solution then agitated
with bicarbonate. After concentration by evaporation it leaves behind the
crude diethylstilbestrol [α,β-(p,p'-dihydroxydiphenyl)-α,β-diethylethylene]
which, when recrystallized from benzene, melts at 170°C to 171°C. The yield
amounts to 75% of the calculated. The total yield of diethylstilbestrol,
calculated on p-hydroxypropiophenone, is 68% of the theoretical.
Stilbestrol (Tablicaps); Stilbetin (Bristol-Myers Squibb);Distilbene;Oestro-gynedron;Stilphostrol.
World Health Organization (WHO)
Diethylstilbestrol, a synthetic estrogen which is a stilbene
derivative, was introduced into obstetric practice in the late 1940s and
subsequently widely used for the treatment of threatened abortion. This use was
later shown to be associated with an increased risk of vaginal cancer in the
offspring which resulted in restrictive regulatory action in several countries.
Diethylstilbestrol and other stilbenes remain available in many countries, however,
for the treatment of certain hormone-dependent neoplasms including carcinoma of
the prostate and postmenopausal breast cancer.
(Reference: (WHODI) WHO Drug Information, 77.1, 16, 1977)
Diethylstilbestrol is an odorless tasteless white crystalline powder. (NTP, 1992)
Diethylstilbestrol is incompatible with strong oxidizing agents, strong bases, acid chlorides and acid anhydrides .
Flash point data for Diethylstilbestrol are not available; however, Diethylstilbestrol is probably combustible.
Diethylstilbestrol is a synthetic estrogen with carcinogenic properties. Causes renal clear-cell carcinoma in Syrian hamster. In humans it causes increased risk of breast cancer, clear cell adenocarcinoma (CCA) of the vagina and cervix, and reproductive anomalies. Used in the treatment of prostate cancer to block the production of testosterone.
These include sodium retention and oedema,nausea,gynaecomastia and impotence in men, and venous and arterial thrombosis. It can cause bone pain and hypercalcaemia when used for breast cancer.
Confirmed carcinogen
producing skin, liver, and lung tumors in
exposed humans as well as uterine and other
reproductive system tumors in the female
offspring of exposed women. Experimental
carcinogenic, neoplas tigenic, tumorigenic,
and teratogenic data. A transplacental
carcinogen. A human teratogen by many
routes. Poison by intraperitoneal and
subcutaneous routes. It causes glandular
system effects by sktn contact. Human
reproductive effects by ingestion: abnormalspermatogenesis; changes in testes, epididymis, and sperm duct; menstrual cycle
changes or disorders; changes in female
ferulity; unspecified maternal effects;
developmental abnormalities of the fetal
urogenital system; germ cell effects in
offspring; and delayed effects in newborn.
Implicated in male impotence and
enlargement of male breasts. Other
experimental reproductive effects.
Mutation data reported. When heated to
decomposition it emits acrid smoke and
fumes. See also ETHINYL ESTRADIOL.
DES had been used extensively as agrowth promoter for cattle and sheep and is used in veterinary medicine to treat estrogen-deficiency disorders. It hasbeen used in humans to prevent spontaneous abortions; totreat symptoms associated with menopause, menstrual disorders, postpartum breast engorgement, primary ovarianfailure, breast cancer, and prostate cancers in males. In1979, the DFA revoked all use of DES in food-producinganimals. The Court of Appeals upheld the Commissioner’sdecision to revoke the use of DES in all food-producing animals on November 24, 1980; the motion to reconsider wasdenied on December 24, 1980.
Skin Contact: Flood areas of body that havecontacted the substance with water. Do not wait to removecontaminated clothing; do it under the water stream. Usesoap to help assure removal. Isolate contaminated clothingwhen removed to prevent contact by others. Eye Contact:Remove any contact lenses at once. Flush eyes well withcopious quantities of water or normal saline for at least20-30 min. Seek medical attention. Inhalation: Leave contaminated area immediately, breathe fresh air. Proper respiratory protection must be supplied to any rescuers. Ifcoughing, difficult breathing, or any other symptomsdevelop, seek medical attention at once even if symptomsdevelop many hours after exposure. Ingestion: If convulsions are not present, give a glass or two of water or milk todilute the substance. Assure that the person’s airway isunobstructed and contact a hospital or poison center immediately for advice on whether or not to induce vomiting.
Diethylstilbestrol is known to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in humans.
Diethylstilbestrol is a known teratogen and carcinogen.
Experimental studies using transgenic estrogen receptor
knockout animals suggest that binding and activation of the
estrogen receptor is required to elicit diethylstilbestrol
toxicity. Hence, diethylstilbestrol lesions primarily appear in
tissues enriched with estrogen receptors. Diethylstilbestrol
binds to the estrogen receptor with a very high affinity and
forms a complex with the target tissue. The complex then
internalizes in to the cell and translocates to the nucleus. Once
in the nucleus, diethylstilbestrol may directly bind with the
cellular DNA and cause mutations and unscheduled DNA
synthesis. Diethylstilbestrol is also known to induce
aneuploidy.
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Color Code—Green: Generalstorage may be used. Prior to working with DES you shouldbe trained on its proper handling and storage. Store in arefrigerator under an inert atmosphere and protect fromlight. A regulated, marked area should be established wherethis chemical is handled, used, or stored in compliance withOSHA Standard 1910.1045.
The name of this material is not on the DOT listof materials for label and packaging standards. However,based on regulations, it may be classified as anEnvironmentally hazardous substances, solid, n.o.s. Thischemical requires a shipping label of “CLASS 9.” It falls inHazard Class 9 and Packing Group III.[20,21]
Diethylstilbestrol’s production and use in biochemical
research, medicine, and veterinary medicine may result in its
release to the environment through various waste streams. It
may also be released to the environment during transport,
storage, or disposal. If released to soil, diethylstilbestrol is
predicted to strongly adsorb to the soil. Volatilization from
the dry or wet soil surface would probably be unlikely. The
extent of biodegradation in soil is not known, although
diethylstilbestrol has been shown to be resistant to degradation
in activated sludge. If released to water, diethylstilbestrol
may bioconcentrate in aquatic organisms and strongly adsorb
to suspended solids and sediments. Diethylstilbestrol is expected
to be essentially nonvolatile on water surfaces.
Diethylstilbestrol would not be susceptible to hydrolysis. The
extent of biodegradation in natural waters is not certain,
although diethylstilbestrol has been shown to be resistant to
degradation in activated sludge. If released to the atmosphere,
diethylstilbestrol vapors should rapidly oxidize, primarily by
reaction with ozone. It is expected to exist solely in the
particulate phase in an ambient atmosphere. Particulatephase
diethylstilbestrol may be removed from the air by wet
and dry deposition.
Clinical claims and research
At first glance, it might be surprising that synthetic nonsteroidal molecules such as diethylstilbestrol (DES) could have the same activity as estradiol or other estrogens. DES can be viewed, however, as a form of estradiol with rings B and C open and a six-carbon ring D. The activity of DES analogs was explained in 1946. It was proposed that the distance between the two DES phenol OH groups was the same as the 3-OH to 17-OH distance of estradiol; therefore, they could both fit the same receptor. Medicinal chemists have shown the OH-to-OH distance to be actually 12.1 ? in DES and 10.9 ? in estradiol. In aqueous solution, however, estradiol has two water molecules that are hydrogen bonded to the 17-OH. If one of the two water molecules is included in the distance measurement, there is a perfect fit with the two OH groups of DES. This suggests that water may have an important role for estradiol in its receptor site.
It is now generally accepted that the estrogens must have a phenolic moiety for binding, but some investigators propose that the receptor may be flexible enough to accommodate varying distances between the two key hydroxyls. This point about estrogens needing a phenolic ring for high-affinity binding to the ER is critical. Steroids with a phenolic A ring and related phenolic compounds lack high-affinity binding to the other steroid hormone receptors.