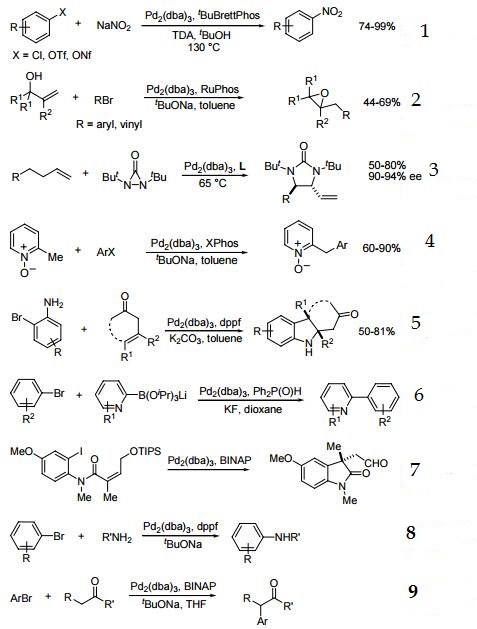
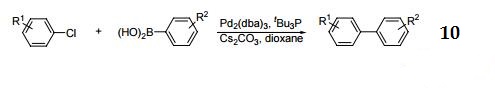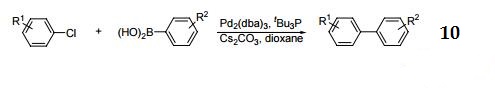1. Catalyst precursor for conversion of aryl chlorides, triflates, and nonaflates to nitroaromatics.
2. Catalyst for the synthesis of epoxides.
3. Catalytic asymmetric allylic and homoallylic diamination of terminal olefins.
4. Site-selective benzylic sp3 palladium-catalyzed direct arylation.
5. Palladium-catalyzed one-pot synthesis of tricyclic indolines.
6. Active catalyst for the Suzuki-Miyaura coupling of 2-pyridyl nucleophiles.
7. Catalyst in combination with BINAP for the asymmetric Heck Arylation of olefins.
8. Precursor for palladium-catalyzed carbon-nitrigen bond formation.
9. Catalyst for α-arylation of ketones,
10. Cross-coupling of aryl halides with aryl boronic acids.