1-Methylpiperazine: synthesis, applications and safety
General Description
The green synthesis of 1-Methylpiperazine is achieved in two steps, starting with an aminolysis reaction to form an intermediate, followed by hydrogenation using Raney nickel, yielding high purity and efficiency with minimal by-products. This cost-effective method boasts high conversion and selectivity rates, enhancing its economic and environmental appeal. As a versatile chemical, 1-Methylpiperazine is used in various industries, notably in lignin recovery and pharmaceuticals, promoting sustainable practices. However, it poses serious health risks, including liver damage, skin burns, and allergic reactions, necessitating stringent safety measures such as proper ventilation, protective gear, and thorough training for those handling it to prevent exposure-related injuries.

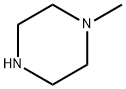
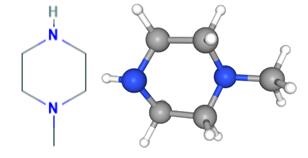
Figure 1. 1-Methylpiperazine
Synthesis
The synthesis of 1-Methylpiperazine leverages a green and cost-effective approach involving two primary steps. Initially, di-Me oxalate reacts with N-methylethylenediamine through an aminolysis reaction to form 1-methylpiperazine-2,3-dione as an intermediate. This step showcases the use of affordable raw materials and a straightforward process, enhancing the method's economic viability and simplicity. Subsequently, this intermediate is subjected to a hydrogenation reaction catalyzed by Raney nickel at temperatures ranging between 150-200°C, under pressures of 3.0-5.0MPa. The catalyst choice and reaction conditions are pivotal for achieving high efficiency and selectivity, leading to the production of 1-Methylpiperazine with impressive purity and yield. The method's merits are manifold: it uses less expensive starting materials, reduces production costs, ensures a cleaner reaction pathway, and simplifies post-processing. Moreover, it minimizes by-product formation, enhancing reaction safety and selectivity. The reported conversion rate of N-methylethylenediamine is 98.34%, with 1-Methylpiperazine selectivity at 96.72%, and an overall yield reaching 95.11%. These figures underscore the process's efficiency, making it a promising route for the eco-friendly synthesis of 1-Methylpiperazine, aligning with the trend towards sustainable industrial practices. 1
Applications
1-Methylpiperazine is a versatile organic compound with applications in the chemical industry as a solvent and intermediate. Its notable use in lignin recovery from biomass enhances the valorization of lignocellulosic materials, contributing to sustainable chemical feedstock production. It also plays a role in pharmaceuticals, aiding in the synthesis of various drugs. Additionally, 1-Methylpiperazine can be employed in epoxy curing agents, improving material properties in industrial processes. Its emergence as a candidate in solvent selection for lignin extraction underlines its potential in green chemistry and renewable resource processing. 2
Safety
1-Methylpiperazine is a chemical compound utilized in various industrial applications, which, however, poses several health risks upon exposure. As an occupational hepatotoxin, repeated or prolonged exposure to this substance can lead to liver damage, with evidence from human poisoning cases and animal studies indicating its potential hepatotoxic effects. It is also classified as a dermatotoxin; it can cause severe skin burns upon direct contact. Furthermore, 1-Methylpiperazine is a known skin sensitizer, meaning it has the capacity to provoke allergic reactions in the skin after exposure. Inhalation of this substance is particularly hazardous, as it can result in toxic pneumonitis—an inflammatory response in the lungs caused by inhaling metal fumes or toxic gases. Studies on rabbits have shown acute dermal exposure leads to skin corrosion, while mice exposed through inhalation experienced convulsions. Oral ingestion in mice has demonstrated multiple adverse effects, including liver damage, renal tubule alterations, peripheral motor nerve disturbances, and general anesthesia. Safety data sheets warn that 1-Methylpiperazine can cause corrosive injuries to both the skin and respiratory tract, emphasizing that it is harmful by inhalation and skin absorption. Protective measures in occupational settings should include adequate ventilation, use of personal protective equipment such as gloves and respirators, and strict adherence to safety protocols to minimize exposure risks. It is essential for workers handling 1-Methylpiperazine to be well-informed about its hazards and trained in emergency response procedures. 3
Reference
1. Green preparation of N-methylpiperazine. 2022, Patent Number: CN114075152.
2. Kwok TT, Bright JR, Vora SR, Chrisandina NJ, Luettgen CO, Realff MJ, Bommarius AS. Solvent Selection for Lignin Value Prior to Pulping. ChemSusChem. 2020 Jan 9;13(1):267-273.
3. 1-Methylpiperazine. Haz-Map, Information on Hazardous Chemicals and Occupational Diseases.
);You may like
Related articles And Qustion
See also
Lastest Price from 1-Methylpiperazine manufacturers

US $0.00/Kg/Bag2024-04-28
- CAS:
- 109-01-3
- Min. Order:
- 2Kg/Bag
- Purity:
- 99% up, High Density
- Supply Ability:
- 20 tons
US $8.00-0.80/KG2024-03-25
- CAS:
- 109-01-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available