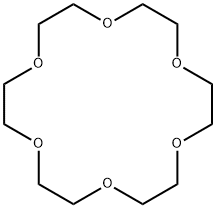
18-Crown-6 coordination compounds: synthesis and properties
General Description
18-Crown-6 coordination compounds display intriguing properties in terms of photoluminescence and nonlinear optics. In the case of Zn2+ ions, which are typically considered "PL inactive," their luminescence is induced through partial exchange with other ions like Mn2+. Surprisingly, ZnI2(18-crown-6) exhibits efficient and unexpected luminescence with a quantum yield of 54%. Computational studies using density functional theory (DFT) and time-dependent density functional theory (TD-DFT) calculations revealed that the luminescence originates from a charge-transfer excitation between halide np orbitals and the empty 4s orbital of the Zn2+ ion. On the other hand, Mn2I4(18-crown-6) (7) demonstrates interesting nonlinear optical properties despite being inversion twins. The crystal displays predominantly linear emission polarization perpendicular to its long axis, following a cos2Θ law. The crystal also exhibits second harmonic signals under laser excitation, indicating potential applications in nonlinear optics. Excitation within a specific range results in orange emission, driven by second harmonic generation-driven excitation and efficient photoluminescence. Overall, 18-crown-6 coordination compounds demonstrate distinct photoluminescence and nonlinear optical properties, making them promising candidates for optoelectronic applications.
Synthesis
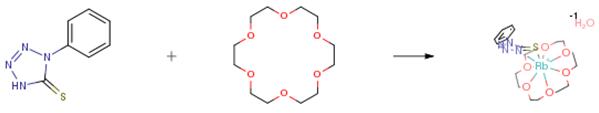
The synthesis of 18-crown-6 coordination compounds involves heating the respective divalent metal halide and the crown ether at mild temperatures (80–150 °C) for 1–3 weeks in an ionic liquid as shown in Figure 1. The use of an ionic liquid is advantageous because it facilitates the dissolution of the metal halides and serves as an inert solvent. It is crucial for the solvent not to coordinate with the dissolved metal cations or form coordination compounds itself under the selected conditions to ensure accurate optical properties. While the ionic-liquid-based synthesis proved optimal for crystal growth, microcrystalline powder samples of some compounds can also be prepared by directly reacting MX2 and 18-crown-6. 1

Figure 1. Synthesis of 18-crown-6 coordination compounds
Properties
Photoluminescence features
The 18-crown-6 coordinated metal halides exhibit remarkable photoluminescence (PL) properties, which can be attributed to their specific structural characteristics. This is particularly surprising for Zn2+ ions, as they are typically considered "PL inactive" and mainly used to induce luminescence through partial exchange with other ions like Mn2+. Additionally, Zn2+ ions were previously known to influence the luminescence of aromatic ligands without directly participating in the emission process. In ZnX2(18-crown-6) complexes, where 18-crown-6 acts as an "innocent" ligand, the crown ether itself does not contribute to the PL phenomenon. This was confirmed by negligible defect-related PL observed in SrI2(18-crown-6) samples. The most noteworthy finding is the efficient and unexpected Zn2+-based luminescence observed in ZnI2(18-crown-6) with a quantum yield of 54%, which is reported here for the first time. To understand the nature of this luminescence, computational studies using density functional theory (DFT) and time-dependent density functional theory (TD-DFT) calculations were conducted on individual ZnX2(18-crown-6) molecules. It was revealed that the first singlet excited state of these compounds originates from a charge-transfer excitation: a transition from a halide np orbital (where n = 3, 4, 5 for X = Cl, Br, I) to the empty 4s orbital of the Zn2+ ion. However, it was also observed that the molecule decomposes in its first singlet excited state, with the halide ion moving away from the rest of the molecule. 1
Nonlinear optical properties
Mn2I4(18-crown-6) (7) exhibits interesting nonlinear optical properties. Despite being inversion twins with a nearly 50:50 ratio of enantiomeric domains, the crystal displays predominantly linear emission polarization perpendicular to its long axis. The emission intensity follows a cos2Θ law, where Θ is the rotation angle of the linear polarizer relative to the long crystal axis. This behavior can be attributed to the unique structural features of Mn2I4(18-crown-6) (7), which has well-separated sensitizer-activator couples and highly anisotropic sites for the sensitizer (MnI1/2I3/1 tetrahedron) and activator (MnI1/2(18-crown-6)). Under laser excitation, the crystal also exhibits second harmonic signals with wavelengths ranging from 480 to 700 nm, indicating incomplete inversion twinning and potential applications in nonlinear optics. Excitation within the range of 960-1160 nm results in orange emission at 605 nm, which is caused by second harmonic generation-driven excitation at 480-580 nm and efficient photoluminescence. These unique properties make Mn2I4(18-crown-6) (7) a promising candidate for optoelectronic applications. 2
Reference
1. Merzlyakova E, Wolf S, Lebedkin S, et al. 18-Crown-6 Coordinated Metal Halides with Bright Luminescence and Nonlinear Optical Effects. J Am Chem Soc, 2021, 143(2):798-804.
2. Shionoya S, Yen WM, Yamamoto H. Phosphor Handbook, CRC Press: Boca Raton, FL, 2006.
);You may like
Related articles And Qustion
See also
Lastest Price from 18-Crown-6 manufacturers

US $0.00-0.00/kg2024-04-26
- CAS:
- 17455-13-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20tons

US $10.00/kg2024-04-24
- CAS:
- 17455-13-9
- Min. Order:
- 1kg
- Purity:
- 99.6%
- Supply Ability:
- 100000