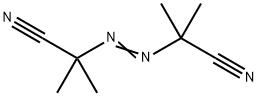
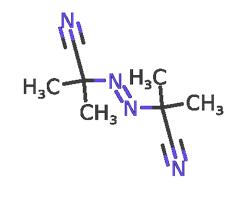
2,2'-Azobis(2-methylpropionitrile): Reactions, synthesis and safety
General description
2,2'-Azobis(2-methylpropionitrile) (abbreviated AIBN) is an organic compound which is often used as a foamer in plastics and rubber and as a radical initiator. As an azo initiator, radicals resulting from 2,2'-Azobis(2-methylpropionitrile) have multiple benefits over common organic peroxides. For example, they do not have oxygenated byproducts or much yellow discoloration. Additionally, they do not cause too much grafting and therefore are often used when making adhesives, acrylic fibers, detergents, etc. 2,2'-Azobis(2-methylpropionitrile) can be used as an initiator for polymerization or copolymerization of vinyl acetate and acrylate. It can also be used as an initiator for the polymerization of monomers such as vinyl chloride, vinyl acetate, and acrylonitrile, and as a foaming agent for rubber and plastics, with a dosage of 10% to 20%. This product can also be used as an intermediate for vulcanizing agents, pesticides, and organic synthesis. Its appearance is as follows:

Figure 1 Appearance of 2,2'-Azobis(2-methylpropionitrile).
Reactions
In its most characteristic reaction, 2,2'-Azobis(2-methylpropionitrile) decomposes, eliminating a molecule of nitrogen gas to form two 2-cyanoprop-2-yl radicals. Because 2,2'-Azobis(2-methylpropionitrile) readily gives off free radicals, it is often used as a radical initiator. This happens at temperatures above 40 °C,[1] but in experiments is more commonly done at temperatures between 66-72 °C.[2] This decomposition has a ΔG of 131 kJ/ mol and results in two 2-cyano-2-propyl (carbon) radicals and a molecule of nitrogen gas. The release of nitrogen gas pushes this decomposition forward due to the increase in entropy. And the 2-cyano-2-propyl radical is stabilized by the -CN group. These radicals formed by the decomposition of 2,2'-Azobis(2-methylpropionitrile) can initiate free-radical polymerizations and other radical-induced reactions. For instance, a mixture of styrene and maleic anhydride in toluene will react if heated, forming the copolymer upon addition of 2,2'-Azobis(2-methylpropionitrile). Another example of a radical reaction that can be initiated by 2,2'-Azobis(2-methylpropionitrile) is the anti-Markovnikov hydrohalogenation of alkenes.
2,2'-Azobis(2-methylpropionitrile) can be used as the radical initiator for Wohl–Ziegler bromination. It decomposes to create the 2-cyano-2-propyl radical, which then abstracts the hydrogen off of tributyltin hydride. This results in a tributyltin radical, which can be used in numerous reactions. For example, this radical could be used to remove a bromine from an alkene.
Synthesis
To a stirred suspension of 1,2-di-1-(1-cyano)-cyclohexylhydrazine (2.46g, 0.01 mol) in water (30mL) was added potassium bromide (1.18g, 0.01 mol) followed by the solution of Oxone® (6.15g, 0.01 mol) in water (20mL) during a period of 30min. The reaction mixture was stirred for additional 3 h and the resultant product was filtered at pump, washed with water and dried under vacuum. The crude product 2,2'-Azobis(2-methylpropionitrile) was recrystallized from petroleum ether. Yield: 1.82 g (75%). Add 50 mL of 95% alcohol to a 150 mL triangular flask equipped with a reflux condenser tube, heat it on a water bath until it is almost boiling, quickly add 5g of 2,2'-Azobis(2-methylpropionitrile), shake to dissolve it completely (boiling time should not be too long, if it is too long, it will decompose seriously), quickly filter the hot solution and cool the filtrate to obtain white crystals, and recrystallize the chemically pure 2,2'-Azobis(2-methylpropionitrile) twice with methanol (1:12), and the purity of the product is about 95%.[3]
Safety
2,2'-Azobis(2-methylpropionitrile) is safer to use than benzoyl peroxide (another radical initiator) because the risk of explosion is far less. However, it is still considered as an explosive compound, decomposing above 65 °C. A respirator dust mask, protective gloves and safety glasses are recommended. Pyrolysis of 2,2'-Azobis(2-methylpropionitrile) without a trap for the formed 2-cyanopropyl radicals results in the formation of tetramethylsuccinonitrile, which is highly toxic. 2,2'-Azobis(2-methylpropionitrile) is a highly toxic substance. The oral LD50 of mice is 17.2-25mg/kg, and the organic cyanide released by thermal decomposition is highly toxic to humans.
References
[1]2,2′-Azobis(2-methylpropionitrile) 441090. (n.d.). Retrieved from https://www.sigmaaldrich.com/catalog/product/aldrich/441090?lang=en?ion=US
[2]Clayden, J., Greeves, N., & Warren, S. (2017). Organic chemistry. MTM.
[3]Tamhankar et al. Oxidation of alkylcyanohydrazines to azo-bis nitriles using Oxone-potassium bromide in aqueous medium. Synthetic Communications (2002), 32(23), 3643-3646.
);You may like
Related articles And Qustion
Lastest Price from 2,2'-Azobis(2-methylpropionitrile) manufacturers

US $141.00/kg2024-02-27
- CAS:
- 78-67-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000 tons

US $20.00-10.00/KG2023-12-11
- CAS:
- 78-67-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200tons