2-Phenyl-2-propyl benzodithioate: properties, synthesis and safety
General Description
2-Phenyl-2-propyl benzodithioate is a versatile compound with unique properties that contribute to its wide range of applications. It is commonly used as a chemical intermediate and plays a crucial role in controlled polymerization processes. Its molecular structure, characterized by hydrogen bond acceptor counts and rotatable bond counts, enhances its flexibility. The compound exhibits lipophilicity with a LogP value of 5, indicating its affinity for lipid-based substances. With specific interactions and structural stability, it proves valuable in various chemical applications. However, caution must be exercised due to its classification as an irritant and potential risks to human health and the environment. Adhering to safety protocols and proper disposal is essential when handling this compound.

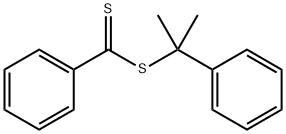
Figure 1. 2-Phenyl-2-propyl benzodithioate
Properties
2-Phenyl-2-propyl benzodithioate, a chemical compound with the formula C16H16S2 and a molecular weight of 272.4 g/mol, is widely used as a chemical intermediate in various fields. It is characterized by its unique properties that contribute to its utility in diverse applications. The molecular structure of 2-Phenyl-2-propyl benzodithioate can be represented by both 2D and 3D conformers. It has no hydrogen bond donor groups but two hydrogen bond acceptor counts and four rotatable bond counts, which enhance its flexibility. Its lipophilicity is indicated by a LogP value of 5. The physical properties of 2-Phenyl-2-propyl benzodithioate include an exact mass of 272.06934286 g/mol, a topological polar surface area of 57.4Ų, and a heavy atom count of 18, contributing to its structural stability. With a formal charge of 0 and a complexity of 271, this compound exhibits specific interactions with other molecules, making it valuable in various chemical applications. Overall, the properties of 2-Phenyl-2-propyl benzodithioate make it a versatile and valuable compound with potential for diverse industrial and research uses. 1
Applications
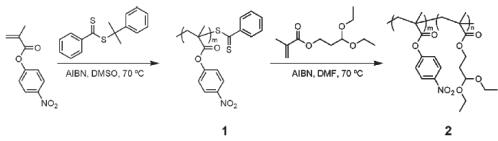
2-Phenyl-2-propyl benzodithioate, a type of reversible addition-fragmentation chain transfer agent, plays a crucial role in the controlled polymerization and cyclization processes. In the context mentioned, the cyclopolymerization of a bis-methacrylate monomer was achieved by utilizing cumyl dithiobenzoate in the RAFT polymerization. The monomer used in this process was derived from trans-cyclohexanediol and 2-methacryloyloxyethyl isocyanate. The key factor behind the successful formation of a 19-membered ring structure was the steric regulation imposed by the cyclohexane ring and hydrogen bonds. By employing cumyl dithiobenzoate as the RAFT agent, the polymerization reaction was carefully controlled. This allowed for the quantitative cyclization of the monomer, leading to the formation of the desired cyclopolymer. The presence of 2-Phenyl-2-propyl benzodithioate ensured that the polymerization proceeded in a controlled manner, resulting in a well-defined and high-quality product. In summary, the application of 2-Phenyl-2-propyl benzodithioate in the RAFT polymerization process facilitated the controlled polymerization and quantitative cyclization of the bis-methacrylate monomer, ultimately leading to the formation of the desired 19-membered ring cyclopolymer. 2
Safety
2-Phenyl-2-propyl benzodithioate poses potential risks to both human health and the environment, as indicated by its classification as an irritant and its associated GHS hazard statements H302, H400, and H410. It is important to recognize the harmful effects of this compound if swallowed, as well as its toxicity to aquatic life, with short-term and long-lasting consequences. Therefore, precautionary measures such as avoiding ingestion, ensuring proper disposal to avoid environmental contamination, and strictly adhering to safety protocols are crucial when handling 2-Phenyl-2-propyl benzodithioate. For safe management in laboratory settings, vital information regarding the hazards and recommended safety measures for this compound can be found in the Laboratory Chemical Safety Summary (LCSS) datasheet. This resource provides essential guidance for the appropriate handling, storage, and disposal of 2-Phenyl-2-propyl benzodithioate, thereby enabling researchers and laboratory personnel to minimize potential risks associated with its use. By following the outlined safety guidelines and exercising caution, the potential adverse impacts of 2-Phenyl-2-propyl benzodithioate on both human health and the environment can be effectively mitigated. 3
Reference
1. Cumyl dithiobenzoate. National Center for Biotechnology Information, 2023, PubChem Compound Summary for CID 11065602.
2. Ochiai B, Ootani Y, Endo T. Controlled cyclopolymerization through quantitative 19-membered ring formation. J Am Chem Soc. 2008 Aug 20;130(33):10832-10833.
3. (1-Methyl-1-phenyl-ethyl) benzenecarbodithioate. European Chemicals Agency, EC / List no. 662-646-5.
);