2-Pyrrolidinone: Synthesis method and chemical reaction
Description
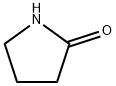
2-Pyrrolidone, also known as α-pyrrolidinone, α-pyrrolidone, γ-aminobutyric lactam, γ-butyrolactam, γ-aminobutyrolactam, butyrolactam and pyrrolidone, is a derivative of γ-butyrolactone. Molecular formula: C4H7NO, molecular weight: 85.1045.
Chemical reaction
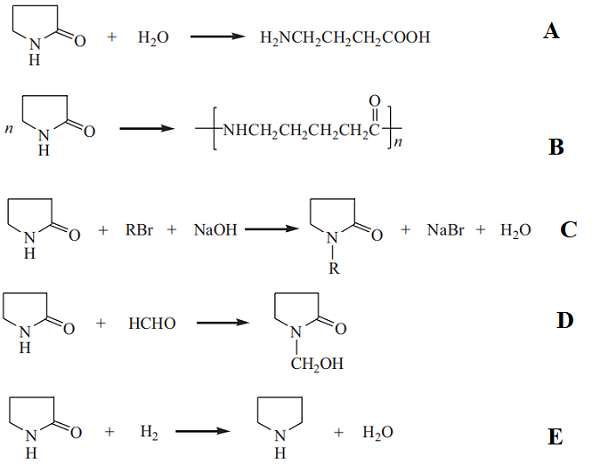
In an aqueous strong acid or base solution, 2-pyrrolidone is hydrolyzed to form 4-aminobutyric acid (A). In the presence of a base, 2-pyrrolidone can be ring-opened and polymerized to form polypyrrolidone(B). Polypyrrolidone is also known as nylon 4, which is a polymer with potential applications in textile fibers, film-forming materials, and molding compounds. The hydrogen atom bonded to the nitrogen atom in the molecule of 2-pyrrolidone is relatively active and is liable to undergo a substitution reaction or a double bond addition. For example, in the presence of a base, 2-pyrrolidone can react with an alkyl halide or a dialkyl sulfate, and the hydrogen connected to the nitrogen atom is replaced by an alkyl group to produce an N-alkyl pyrrolidone (C). 2-Pyrrolidone undergoes an addition reaction with the carbonyl group of an aldehyde to produce N-hydroxyalkyl pyrrolidone (D). The carbonyl group of 2-pyrrolidone is hydrogenated to yield pyrrolidine in the presence of a cobalt catalyst at a high temperature(E).
Synthesis method
Commercial 2-pyrrolidone of industrial grade requires a purity of more than 99.5% and a moisture content of less than 0.1%. There are several processes for the production of 2-pyrrolidone in industry, including hydrogenation and amination of maleic anhydride or succinic acid, ammonolysis of γ-butyrolactone in the vapor phase, and ammonolysis of γ-butyrolactone in the liquid phase. However, the most widely used process is the ammonolysis of γ-butyrolactone in the liquid phase[1].
γ-Butyrolactone and liquid ammonia (or aqueous ammonia) are reacted in the liquid phase at a temperature of 250 to 290 °C and a pressure of 8.0 to 16.0 MPa to produce 2-pyrrolidone with high conversion and selectivity:
The reaction is carried out in the absence of a catalyst. Generally, a tubular reactor operated in continuous mode is used to produce 2-pyrrolidone. The molar ratio of γ-butyrolactone/NH3/H2O is 1: (2.2 to 3): (1.6 to 2.3). The residence time of the reaction mixture in the tubular reactor is usually from 20 to 120 min. The conversion of γ-butyrolactone is close to 100%, and the selectivity to 2-pyrrolidone is higher than 94%. The presence of water in the reaction system improves the selectivity, as in the vapor phase ammonolysis process. The excess and unreacted ammonia is distilled off in a distillation tower and sent back to an absorption tower to be absorbed to a certain concentration for recycling to the reaction system.
References
[1] Li, Sifang. “Manufacture of Fine Chemicals from Acetylene.” 2021. 0.
);You may like
Related articles And Qustion
Lastest Price from 2-Pyrrolidinone manufacturers

US $10.00/kg2023-09-07
- CAS:
- 616-45-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 500t/month

US $500.00/KG2023-08-16
- CAS:
- 616-45-5
- Min. Order:
- 1KG
- Purity:
- >99%
- Supply Ability:
- 50000kg/Month