4-Hydroxybenzaldehyde: Synthesis, applications and bioactivities
General description
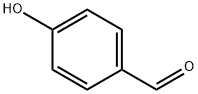
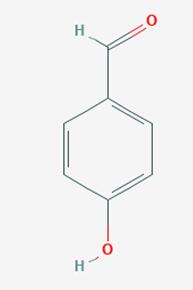
4-Hydroxybenzaldehyde (4-HBA) is a naturally occurring benzaldehyde and the major active constituent of Gastrodia elata, which has long been used as a Chinese herbal medicine to treat headaches, migraines, as well as some neuralgias and nervous disorders. 4-Hydroxybenzaldehyde is a structural isomer of salicylaldehyde with one hydroxyl (-OH) group at the para position of the phenolic ring. Several studies have suggested that 4-Hydroxybenzaldehyde is an active candidate for improving insulin resistance and inhibiting cholinesterase [1]. The catalytic oxidation method of p-cresol is the synthesis of 4-Hydroxybenzaldehyde by directly oxidizing p-cresol with air or oxygen under the action of a catalyst. In the 1980s, Japan, the United States, Germany, and others conducted in-depth research and reporting on this process route. In the late 1980s and early 1990s, several research and production units in Jiangsu, Shanghai, Dalian, and other places in China also conducted research and development on this process, and used it for industrial production. Its appearance is as follows:

Figure 1 Appearance of 4-Hydroxybenzaldehyde
Synthesis
By using the p-nitrotoluene oxidation-reduction method, p-nitrotoluene is synchronously oxidized and reduced with sodium polysulfide to obtain 4-Hydroxybenzaldehyde. The specific process is as follows: Mix p-nitrotoluene, ethanol solvent, and surfactants (such as OP Tween) in a mass ratio of 1:5:0.02-0.04 evenly, add sodium polysulfide aqueous solution dropwise at 80-85 ℃, and react for 2-3 hours. The product was distilled with steam to remove p-nitrotoluene and p-aminotoluene. Extracting p-aminobenzaldehyde with ether. The conversion rate and yield of the reaction are both above 90%. Sodium polysulfide can be prepared from sodium hydrosulfide, caustic soda, and sulfur as raw materials. Treat p-aminobenzaldehyde with 40% sulfuric acid, add a 30% sodium nitrite solution at 0-3 ℃, and react for about 30 minutes. Use a small amount of urea to decompose the excess sodium nitrite to obtain a diazonium salt solution of p-aminobenzaldehyde. This solution is hydrolyzed in the presence of sulfuric acid at a temperature of 80-85 ℃ for about 30 minutes. The product was extracted, purified, and dried to obtain a 4-Hydroxybenzaldehyde product with a yield of over 90%.
Applications
4-Hydroxybenzaldehyde is an important intermediate in medicine, spices, and liquid crystals. It can react with dimethyl sulfate to produce anisaldehyde, react with acetaldehyde to produce p-hydroxycinnamaldehyde, and further oxidize to produce cinnamic acid. This product can be directly oxidized to produce p-hydroxybenzoic acid, reduced to p-hydroxybenzyl alcohol, and can be used as spices; At the same time, 4-Hydroxybenzaldehyde is also an important pharmaceutical intermediate, liquid crystal raw material, and other organic synthesis intermediates, with a wide range of uses. In addition to being directly used as a spice, 4-Hydroxybenzaldehyde is also used as an intermediate in the production of other spices, pharmaceutical raw materials, and in the production of dehydrepinephrine hydrochloride, adrenaline, quinine, etc. At the same time, it can be used as a nickel plating brightener, chemical analysis reagent (sugar quantitative analysis), photographic emulsion, and bactericide.
Bioactivities
4-Hydroxybenzaldehyde, an analogue of p-hydroxybenzyl alcohol, showed an inhibitory effect on the GABA transaminase, and its inhibitory activity was higher than that of valproic acid, a known anticonvulsant. In the brain of PTZ-treated rats, brain lipid peroxidation was significantly increased, while it recovered to the control level after treatment with 4-hydroxybenzaldehyde. It may be concluded that antioxidation and positive modulation of GABAergic neuromodulation of 4-Hydroxybenzaldehyde partially contribute to an antiepileptic and anticonvulsive activity of Gastrodia elata B1 [2]. In addition, using an in vitro approach, it found that 4-HBA significantly promoted keratinocyte cell migration and invasion by increasing focal adhesion kinase and Src activity. Moreover, 4-Hydroxybenzaldehyde treatment also promoted wound healing and re- epithelialization in an in vivo excision wound animal model. Combination treatment with 4-Hydroxybenzaldehyde and platelet-derived growth factor subunit B homodimer showed synergistic effects in promoting wound healing. Taken together, it demonstrated that treatment with 4-Hydroxybenzaldehyde promoted keratinocyte migration and wound healing in mouse skin through the Src/mitogen-activated protein kinase pathway [3].
References
[1]Tang, W. & Eisenbrand, G. Chinese drugs of plant origin: chemistry, pharmacology, and use in traditional and modern medicine. (Springer-Verlag, 1992).
[2]Ha J H, Lee D U, Lee J T, et al. 4-Hydroxybenzaldehyde from Gastrodia elata B1. is active in the antioxidation and GABAergic neuromodulation of the rat brain[J]. Journal of Ethnopharmacology, 2000, 73(1-2): 329-333.
[3]Kang C W, Han Y E, Kim J, et al. 4-Hydroxybenzaldehyde accelerates acute wound healing through activation of focal adhesion signalling in keratinocytes[J]. Scientific reports, 2017, 7(1): 1-11.
);You may like
Related articles And Qustion
Lastest Price from 4-Hydroxybenzaldehyde manufacturers

US $0.00/kg2024-04-30
- CAS:
- 123-08-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 500mt

US $11340.00-11320.00/Tons2024-04-30
- CAS:
- 123-08-0
- Min. Order:
- 10Tons
- Purity:
- 99.99%
- Supply Ability:
- 100Tons