A bifunctional catalyst: L-Proline
Proline, L-proline, and D-proline
The application of L-Proline Proline is a non-essential amino acid that is synthesized from glutamic acid. It is an essential component of collagen and is necessary for properly functioning joints and tendons. Only the L-enantiomer is found in nature.
L-proline, an amino acid, could be biosynthesized by humans and other animals. In vivo, it is mainly from another non-essential amino acid, L-glutamic acid. L-Proline is a metabolite found in or produced by Escherichia coli.
L-proline and D-proline are often used as asymmetric organocatalysts for various organic reactions due to their conformational rigidity compared to other amino acids. Where L-proline is a natural amino acid, D-proline is an unnatural amino acid, with one basic and one acidic center each[1].
Source
L-Proline can be obtained through dietary, plant-based, and animal sources. Plant-based sources include soy products, legumes, nuts, and certain grains. Some animal-based sources include meat, dairy products, and eggs.
Organocatalyst
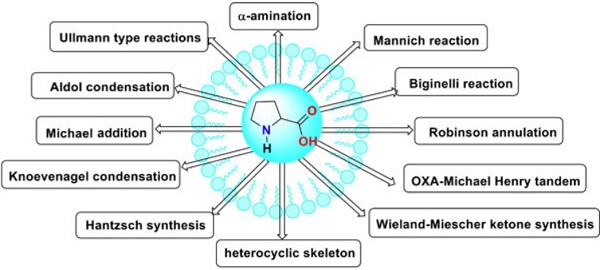
L-proline is a natural amino acid having secondary amine functionality and acts as a bifunctional catalyst (organo-catalyst). The amino-functional group acts as Lewis base type while carboxylic acids act as Brønsted acid type catalysts. It catalyzed different asymmetric syntheses, including known reactions such as Aldol condensation, Mannich reaction, Michael Addition, Knoevenagel condensation, Hantzsch synthesis, OXA-Michael Henry tandem, Ullmann reactions, Wieland-Miescher ketone synthesis, Robinson annulation, Biginelli reaction, α- amination. It is also an essential catalyst for synthesizing heterocyclic skeletons such as coumarin, spiro-oxindoles, imidazoles, benzimidazoles, quinoxalines, podophyllotoxin, benzothiazoles, isoxazolidines, phenothiazines, aziridine, indole, 1,5-benzodiazepines, pyridine, and quinazolines[2-3].
As an efficient chiral small-molecule organocatalyst, It could be used for the direct asymmetric aldol reaction between unmodified acetone and various aldehydes. Bhattacharjee et al. Synthesieze chromeno[2,3-d] pyrimidine- triones, xanthenes in water with l-proline as organo catalyst. This reaction exhibited the efficient asymmetric induction property of the organocatalyst l-proline[4].
References
[1] Jun Wang. "Capillary array electrophoresis for the research of asymmetric transformation reaction of L-proline to D-proline." Journal of separation science 28 18 (2005): 2551–2554.
[2] ThoratBapu R. "L-Proline: A Versatile Organo-Catalyst in Organic Chemistry." Combinatorial chemistry & high throughput screening 26 6 (2023): 1108–1140.
[3] Deboshikha Bhattacharjee. "l-proline as an efficient asymmetric induction catalyst in the synthesis of chromeno[2,3-d]pyrimidine-triones, xanthenes in water." Tetrahedron 73 25 (2017): Pages 3497-3504.
[4] ZhangCuizhi. "Polyacrylic Acid Supported L-proline as an Effective Heterogeneous Catalyst for the Direct Asymmetric Aldol Reaction." Current organic synthesis (2023).
);You may like
Related articles And Qustion
See also
Lastest Price from L-Proline manufacturers

US $0.00/kg2024-04-20
- CAS:
- 147-85-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000kg

US $6.00/KG2024-04-19
- CAS:
- 147-85-3
- Min. Order:
- 1KG
- Purity:
- More than 99%
- Supply Ability:
- 2000KG/MONTH