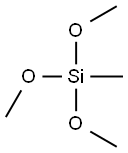
A immediate product:Methyltrimethoxysilane
General description
With the rapid development of domestic silicone industry, the consumption of silicone rubber and silicone resin is also increasing. As an important component of silicone rubber and silicone resin, the consumption of methyltrimethoxysilane (MTMS) is also increasing. However, the old batch process has long reaction time, unstable reaction process, low raw material conversion and high production cost, which seriously restricts the production capacity of methyltrimethoxysilane in China. Therefore, developing new and efficient new processes is the fundamental way to solve this situation[1].
Application and Pharmacology
Methyltrimethoxysilane (MTMS)-based silica–iron oxide superhydrophobic nanocomposites. a facile synthesis of superhydrophobic silica–iron oxide nanocomposites via a co-precursor sol–gel process. The choice of the silica precursor (Methyltrimethoxysilane, MTMS) in combination with iron nitrate altered the pore structure dramatically. The influence of iron oxide doping on the structural properties of pristine MTMS aerogel is discussed. uch gels are inherently hydrophilic, as they do not carry any nonpolar organic groups. Hydrophobic tri-functional alkoxysilane precursors such as Methyltrimethoxysilane (MTMS) or Methyltriethoxysilane (MTES) on the other hand come with a nonreactive methyl group. This leads to an intrinsic hydrophobic character of the precursor and the resulting gels. At the same time, the average connectivity in the gel is reduced, since only three alkoxy groups are available for assembly of the silica network structure. Rao and coworkers have demonstrated the ability to create intrinsically superhydrophobic silica aerogels from MTMS and MTES precursors with a stable network structure[2].
Methyltrimethoxysilane (MTM) as a Reagent for Direct Amidation of Carboxylic Acids, Methyltrimethoxysilane [MTM, CH3Si(OMe)3] has been demonstrated to be an effective, inexpensive, and safe reagent for the direct amidation of carboxylic acids with amines. Two simple workup procedures that provide the pure amide product without the need for further purification have been developed. The first employs an aqueous base-mediated annihilation of MTM. The second involves simple product crystallization from the reaction mixture providing a low process mass intensity direct amidation protocol[3].
Figure 1 an effective, inexpensive, and safe reagent for the direct amidation of carboxylic acids with amines
Particleboard is a very important wood composite product used in furniture manufacturing, but the phenomena of warping deformation, thickness swelling and delamination are occurrence frequently while it is used in changing climates and in contact with liquid water or oil. In order to improve the humidity resistance and dimensional stability of particleboard, methyltrimethoxysilane (MTMS) was mixed with 0.1 mol/L hydrochloric acid in a 4∶1 v/v ratio and the mixture was then sonicated in an ice-bath for 360 minutes to induce hydrolysis. After hydrolysis, particleboards were immersed in the hydrolyzed MTMS solutions for 5 minutes. The properties of particleboards before and after coated with MTMS were measured and analyzed which were the wetting properties, ageing resistance, surface average roughness, surface chemical structure, physical and mechanical properties. The results showed that the particleboard coated with MTMS displayed a stable repellence for water and oil liquids and had ageing resistance. Furthermore, the dimensional stability, surface density and mechanical performance of modified particleboard were improved at the same time.
In the presented research, two trialkoxysilanes were used to investigate their reactivity with microcrystalline cellulose (MCC) applied as a model material. As a continuation of the previous study, the research aimed at evaluation of the durability and potential reversibility of the silane treatment. Two different solvents and a mixture thereof were used for cellulose modification. The influence of amino group/pH, an excess of silanes and re-soaking with water on binding with cellulose was examined. The results obtained confirm that both selected silanes can effectively modify MCC. However, the treatment with 3-(2-aminoethylamino)propyltrimethoxysilane occurred more effective than with Methyltrimethox ysilane due to the presence of amino groups. Among the three tested solvents, the most effective was pure water. In con trast, the use of ethanol and a mixture of ethanol and water gave significantly worse results. Summarising, the presented research clearly shows how important the type of the functional group in alkoxysilanes is for its chemical reactivity with natural polymers, which is crucial for their application in waterlogged wood conservation.
Synthesis
Technical principle: the vaporized methanol and methyltrichlorosilane are esterified and stripped in the double tower, neutralized and rectified to finally obtain the finished product of methyltrimethoxysilane. Performance index: main content: ≥ 99.0%; Methanol content: ≤ 0.5%; Chloride ion content: ≤ 10ppm in this preparation method, the vaporized methanol is contacted with methyltrichlorosilane countercurrent for esterification reaction, and the integrated design of double tower reaction and purification is adopted, which not only makes the reaction more rapid and sufficient, but also effectively removes the HCl produced by the reaction. This process has the advantages of high production efficiency, stable product quality, simple operation and easy DCS computer control. Our company has used this technology for mass production, and the output and quality of products are significantly improved compared with batch method. The application of this technology in production has achieved the expected effect, but a large amount of HCl gas is produced in the production process of this product, which causes great environmental pollution[4,5].
References
1.Braddock D. C., Davies J. J. & Lickiss P. D., "Methyltrimethoxysilane (MTM) as a Reagent for Direct Amidation of Carboxylic Acids," Organic Letters, Vol.24, No.5(2022), pp.1175-1179.
2.Nadargi D., Gurav J. & Marioni M. A. et al., "Methyltrimethoxysilane (MTMS)-based silica–iron oxide superhydrophobic nanocomposites," Journal of Colloid and Interface Science, Vol.459(2015), pp.123-126.
3.Pietras P., Maciejewski H. & Mazela B., "Reactivity of Microcrystalline Cellulose with Methyltrimethoxysilane and 3-(2-Aminoethylamino) propyltrimethoxysilanes," Acta Chimica Slovenica, Vol.68, No.4(2021), pp.849-860.
4.Xiao Junping, Zhao Jiaxu: preparation process of methyltrimethoxysilane, 2011.
5.Xie linkun, Chai Xijuan, Xu kaimeng, etc.: methyltrimethoxysilane impregnated modified particleboard and its performance analysis, forest products industry, 2021, No. 02, pp. 1-6.
);You may like
See also
Lastest Price from Methyltrimethoxysilane manufacturers
US $8.00-0.60/KG2024-03-25
- CAS:
- 1185-55-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available

US $0.00/KG2023-09-11
- CAS:
- 1185-55-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg