A material with many uses: Natural rubber
Description
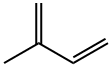
Natural rubber (NR; polyisoprene) or cis-1,4-polyisoprene has a basic monomer unit, a cis-1,4-isoprene (sometimes called caoutchouc). In Myanmar, natural rubber (Hevea brasiliensis) is mainly grown in the southern part of the country, where the rainfall is too high, leading to the suspension of tapping in the rainy season and intensive tapping after the rainy season[1].

Preparation method
Natural rubber is made by processing the sap of the rubber tree (i.e., Hevea brasiliensis) with steam and compounding it with vulcanizing agents, antioxidants, and fillers. A desired color can be obtained by incorporating suitable pigments (e.g., red: iron oxide, Fe2 O3, black: carbon black, white: zinc oxide, ZnO).
Uses and limitations
Natural rubber has good dielectric properties, excellent resilience, an elevated damping capacity, and good tear resistance. Generally, natural rubbers are chemically resistant to non-oxidizing dilute mineral acids, alkalis, and salts[2]. However, they are readily attacked by oxidizing chemicals, atmospheric oxygen, ozone, oils, benzene, and ketones, and as a general rule, they also have poor chemical resistance to petroleum and its derivatives and many organic chemicals in which the material softens. Moreover, natural rubbers are highly sensitive to UV irradiation (e.g., sunlight exposure). Hence, natural rubber is a general-purpose material for applications requiring abrasion/wear resistance, electric resistance, and damping or shock-absorbing properties.
Nevertheless, owing to their mechanical limitations, natural and many synthetic rubbers are converted into a harder and more stable product by vulcanization and compounding with additives. The vulcanization process involves mixing crude natural or synthetic rubber with 25 wt.% sulfur and heating the blend at 150°C in a steel mold. Due to the cross-linking reaction between adjacent carbon chains, the resulting rubber material is harder and stronger than the previous raw material. Therefore, industrial applications of natural rubber include components such as internal lining for pumps, valves, piping, hoses, and machined components when hardened by vulcanization. However, because natural rubber has low chemical resistance and is sensitive to exposure to sunlight, unsuitable properties in many industrial applications, it is today replaced by newer, improved elastomers.
References
[1] Zar Ni Zaw, R. Lacote, S. Sdoodee. “Performances of low frequency rubber tapping system with rainguard in high rainfall area in Myanmar.” Australian Journal of Crop Science 317 1 (2017): 1451–1456.
[2] Materials Handbook. DOI 10.1007/978-1-84628-669-8.
);You may like
Lastest Price from POLYISOPRENE manufacturers

US $20.00/kg2024-02-27
- CAS:
- 9003-31-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000 tons

US $1.00/KG2022-08-24
- CAS:
- Isoprene rubber
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20T