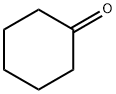
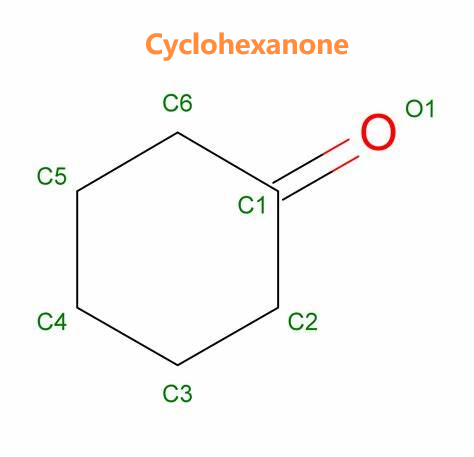
A pharmaceutical intermediate:Cyclohexanone
General description
Cyclohexanone appears as a colorless to pale yellow liquid with a pleasant odor. Less dense than water . Flash point 111°F. Vapors heavier than air. Used to make nylon, as a chemical reaction medium, and as a solvent. Cyclohexanone is a cyclic ketone that consists of cyclohexane bearing a single oxo substituent. It has a role as a human xenobiotic metabolite. Cyclohexanone (also known as oxocyclohexane, pimelic ketone, ketohexamethylene, cyclohexyl ketone or ketocyclohexane) is a six-carbon cyclic molecule with a ketone functional group. It is a colorless, oily liquid with an acetone-like smell.
Application and pharmacokinetics
Cyclohexanone is an important chemical raw material and the main intermediate for the manufacture of nylon, caprolactam and adipic acid. It is also an important industrial solvent, such as paint, especially those containing nitrocellulose, vinyl chloride polymer and its copolymer or methacrylate polymer paint. It is used as an excellent solvent for organophosphorus pesticides and many analogues, as a solvent for dyes, as a viscous solvent for piston aviation lubricating oil, and as a solvent for grease, wax and rubber. It is also used as homogenizing agent for dyeing and fading silk, degreasing agent for polishing metal, wood coloring and painting. Used as a high boiling point solvent for nail polish and other cosmetics. It is usually prepared into mixed solvent with low boiling point solvent and medium boiling point solvent to obtain appropriate volatilization speed and viscosity.
Neurodevelopment was assessed at age 12 months with the Bayley Scales of Infant and Toddler Development III, assessing cognitive, language, and motor function composite scores standardized to a population mean (SD) of 100 (15). Linear regression models were used to determine covariate-adjusted differences in 12-month cognitive, language, and motor composite scores per interquartile range increase in cyclohexanone level or summed metabolite molar concentrations. Among 85 included neonates, mean (SD) age at surgical treatment was 9.7 (5.3) days, 49
(58%) were boys, and 54 (64%) underwent corrective repair. Mean (SD) Bayley Scales of Infant and Toddler Development III composite scores were 108.2 (12.2) for cognitive function, 104.7 (11.0) for language function, and 94.7 (15.7) for motor function. Median (interquartile range) cyclohexanone levels increased approximately 3-fold from immediately prior to surgical treatment to immediately after surgical treatment.
In humans, cyclohexanone is metabolized to cyclohexanol, which is conjugated with glucuronic acid and excreted mainly in urine, where very little cyclohexanone or cyclohexanol is found. The metabolism and kinetics of cyclohexanone were studied in a group of volunteers (4 males and 4 females) during and after 8-h inhalation exposures to 101, 207, and 406 mg/m3. Following exposure to the highest dose, the metabolic yields of urinary cyclohexanol, 1,2- and 1,4- cyclohexanediol and their glucuronide conjugates were 1%, 39%, and 18%, respectively. The elimination half-times (t½) of the 1,2- and 1,4-diols, were 16 h and 18 h, respectively. Consequently, after repeated exposure over 5 days, there was no accumulation of urinary cyclohexanol, whereas there was cumulative excretion of the diols. The permeation rate of cyclohexanone liquid through the skin was 37–69 mg/cm2hour, indicating that occupational exposure by this route is of minor importance[1].
The targeting of pro-inflammatory enzymes becomes a therapeutic intervention when acute inflammation is proliferating in pathological conditions. This research is intended to carry out an evaluation of inhibiting and inducing enzymes with inflammatory associations with 28 cyclohexanone analogs based on the ligustrazine. Tests were undertaken with inhibitor screening assay kits using a range of synthetic compounds to investigate how they could inhibit the activity of cyclooxygenase (COX) enzymes, secretory phospholipase A2(sPLA2), and lipoxygenase (LOX) enzyme. Significant and similar inhibitory activities against sPLA2with were noted with
synthetic compounds which included1fand 1g(IC50= 2.2μM). The optimal inhibitory activity regarding LOX enzyme was shown with compounds1d(IC50= 8.1μM) and1e(IC50= 7.5μM).Additionally, the compounds1b, 1d, 1e, 2n,and2owere shown to be significant inhibitors of COX-1 activity with IC50values 0.09 to 0.7μM. The outcomes of assays for COX inhibition demonstrated that the same compounds had a further strong inhibitive influence on the COX-2 enzyme[2].
Figure1. Diagram for Structure–activity relationship studies.
Synthesis
In the process of cyclohexanone production by cyclohexane oxidation, a large amount of cyclohexanol will be produced in the process of cyclohexane oxidation and cyclohexyl hydrogen peroxide decomposition. The crude cyclohexanone can be obtained by dehydrogenation of cyclohexanol. In the production process, cyclohexanol steam is heated by cyclohexanol superheater and enters the dehydrogenation reactor from the top of dehydrogenation reactor through distributor. Cyclohexanol steam passes through tubular reactor equipped with catalyst from top to bottom for dehydrogenation reaction. The reaction temperature is controlled at 230 ~ 280 ℃, the pressure is controlled at 0.02 ~ 0.1 MPa, the conversion is controlled at 45% ~ 55%, and the selectivity of cyclohexanone is more than 99%.Dehydrogenation of cyclohexanol to cyclohexanone is a reversible endothermic reaction in thermodynamics. The dehydrogenation of cyclohexanol to cyclohexanone is the main reaction. The dehydrogenation process is accompanied by many side reactions, such as dehydration of cyclohexanol to cyclohexene, aromatization to phenol and dimerization dehydration of cyclohexanone. It can be seen that the advantages and disadvantages of catalysts are the key to the reaction of cyclohexanone dehydrogenation to cyclohexanone. The commonly used catalysts in cyclohexanol catalytic dehydrogenation process are mainly copper based catalysts. The catalytic performance of copper silicon catalyst and copper chromium catalyst for cyclohexanol dehydrogenation. Within the investigated liquid space velocity and reaction temperature, copper silicon catalyst and copper chromium catalyst can ensure better cyclohexanone selectivity, cyclohexanone selectivity is 99.0% ~ 99.3%, and the conversion of cyclohexanol is more than 66%. Among them, under the action of copper silicon catalyst, when the reaction temperature is 230 ℃ and the liquid space velocity is about 0.8 H-1, the better reaction effect can be obtained. The reaction conversion is 77% and the selectivity is 99.4%. Under the action of copper chromium catalyst, when the reaction temperature is 250 ℃ and the liquid space velocity is about 1.2 H-1, a better reaction effect can be obtained. The reaction conversion is 85% and the selectivity is 99.2%[3].
Safety and storage
1.Cyclohexanone was evaluated for genotoxicity, repeated dose toxicity, developmental and reproductive toxicity, local respiratory toxicity, phototoxicity/photoallergenicity, skin
sensitization, and environmental safety. Data show that cyclohexanone is not genotoxic. Data on cyclohexanone provide a calculated MOE > 100 for the repeated dose toxicity and developmental and reproductive toxicity endpoints. The skin sensitization endpoint was completed using the DST for reactive materials (64 μg/cm2); exposure is below the DST. The phototoxicity/photoallergenicity endpoints were evaluated based on UV spectra; cyclohexanone is not expected to be phototoxic/photoallergenic. The local respiratory toxicity endpoint was evaluated using the TTC for a Cramer Class II material, and the exposure to cyclohexanone is below the TTC (0.47 mg/day). The environmental endpoints were evaluated; cyclohexanone was found not to be PBT as per the IFRA Environmental Standards, and its risk quotients, based on its current volume of use in Europe and North America (i.e., PEC/PNEC), are < 1[4].
2.Precautions for transportation: during transportation, the transportation vehicles shall be equipped with corresponding varieties and quantities of fire-fighting equipment and leakage emergency treatment equipment. It's best to transport in the morning and evening in summer. The tank car used in transportation shall be equipped with grounding chain, and a hole diaphragm can be set in the tank to reduce static electricity caused by vibration. It is strictly prohibited to load and transport with oxidants, reducing agents, edible chemicals, etc. During transportation, it shall be protected from sun exposure, rain and high temperature. During stopover, keep away from kindling, heat source and high temperature area. The exhaust pipe of the vehicle carrying this article must be equipped with a fire stop device. It is forbidden to use mechanical equipment and tools that are easy to produce sparks for loading and unloading. During highway transportation, drive according to the specified route, and do not stay in residential areas and densely populated areas. It is forbidden to slip away during railway transportation. It is strictly prohibited to transport in bulk with wooden ships and cement ships; Storage precautions: store in a cool and ventilated warehouse. Keep away from kindling and heat sources. The storage temperature should not exceed 30 ℃. Keep the container sealed. It shall be stored separately from oxidant and reductant, and mixed storage shall not be allowed. Explosion proof lighting and ventilation facilities shall be adopted. It is forbidden to use mechanical equipment and tools that are easy to produce sparks. The storage area shall be equipped with leakage emergency treatment equipment and appropriate receiving materials.
References
1.Everett A. D., Buckley J. P. & Ellis G. et al., "Association of Neurodevelopmental Outcomes With Environmental Exposure to Cyclohexanone During Neonatal Congenital Cardiac Operations," JAMA Network Open, Vol.3, No.5(2020), p.e204070.
2.Alotaibi N. H., Alharbi K. S. & Alzarea A. I. et al., "Pharmacological appraisal of ligustrazine based cyclohexanone analogs as inhibitors of inflammatory markers," European Journal of Pharmaceutical Sciences, Vol.147(2020), p.105299.
3.Jiang Yutu, Fu Wenying, Ma Liyong, etc.: Research on catalytic process for dehydrogenation of cyclohexanol to cyclohexanone, chemical production and technology, No. 06, 2021, pp. 1-3.
4.Api A. M., Belmonte F. & Belsito D. et al., "RIFM fragrance ingredient safety assessment, cyclohexanone, CAS Registry Number 108-94-1," Food and Chemical Toxicology, Vol.138(2020), p.111231.
);You may like
Related articles And Qustion
Lastest Price from Cyclohexanone manufacturers

US $50.00-30.00/kg2024-04-26
- CAS:
- 108-94-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20Tons

US $0.00/KG2023-12-12
- CAS:
- 108-94-1
- Min. Order:
- 1000KG
- Purity:
- 99
- Supply Ability:
- 30TON