Application and Pharmacology of Cinnamic acid
General description
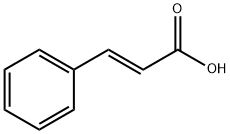
Cinnamic acid is a monocarboxylic acid that consists of acrylic acid bearing a phenyl substituent at the 3-position. It is found in Cinnamomum cassia. It has a role as a plant metabolite. It is a member of styrenes and a member of cinnamic acids. It is a conjugate acid of a cinnamate. Cinnamic acid is a natural product found in Marsypopetalum crassum, Aiouea brenesii, and other organisms with data available. Cinnamic acid is a metabolite found in Escherichia coli.
The role played by cinnamic acid derivatives in treating cancer,bacterial infections diabetes and neurological disordersamong many,has been reported. Cinnamic acid is obtained from cinnamon bark.Its structure is composed of a benzene ring an alkene double bond and an acrylic acid functional group making it possible to modify the aforementioned functionalities with a variety of compounds resulting in bioactive agents with enhanced efficacy. The nature of the substituents incorporated into cinnamic acid has been found to play a huge role in either enhancing or decreasing the biological efficacy of the synthesized cinnamic acid derivatives. Some of the derivatives have been reported to be more effective when compared to the standard drugs used to treat chronic or infectious diseases in vitro,thus making them very promising therapeutic agents.Compound 20 displayed potent anti-TBactivity compound 27 exhibited significant antibacterial activityon.
Application and Pharmacology
1.The World Health Organization (WHO) recognizes infectious diseases caused by bacteria viruses and fungi as a global health threate specially in developing and poor countries. About 3.5 million people die from infectious diseases annually17In 2018the WHO reported 37.9 million people to be living with Human Immunodeficiency Virus (HIV)worldwide with 770000 dying from AlDS-related illnesses181The 2019 Global tuberculosis (TB)reported an estimated 100 milion TE infections and 1.2 million deaths in 2018.Of the TB cases8.6% were people living with HIV. The appearance of drug-resistant microbial strains has led to difficulty in treating diseases such as TB and malaria. Cinnamic acid derivatives are known for their antimicrobial activity and researchers are searching for antimicrobial agents which are more effective than the currently used standard drugs extracted a 4-hydroxycinnamic acid derivativemethyl2-((E)-2-[4-(formyloxy)phenyl ethenyl}-4-methyl-3-oxopentanoate(2)(Figure 2) from a plant-based endophytic fungus Pyronema sp The biological activity of the compound was evaluated in vitro on a non-tuberculous mycobacterium Mycobacterium marinum,responsible for skin infections.The compound exhibited a goodinhibitory effect with an IC5o of 64 uM[1].
2.Cinnamic Acid Conjugates in the Rescuing and Repurposing of Classical Antimalarial Drugs: Cinnamic acids are compounds of natural origin that can be found in many different parts of a wide panoply of plants, where they play the most diverse biological roles, often in a conjugated form. For a long time, this has been driving Medicinal Chemists towards the investigation of the therapeutic potential of natural, semi-synthetic, or fully synthetic cinnamic acid conjugates. These efforts have been steadily disclosing promising drug leads, but a wide chemical space remains that deserves to be further explored. Amongst different reported approaches, the combination or conjugation of cinnamic acids with known drugs has been addressed in an attempt to produce either synergistic or multi-target action. In this connection, the present review will focus on efforts of the past decade regarding conjugation with cinnamic acids as a tool for the rescuing or the repurposing of classical antimalarial drugs, and also on future perspectives in this particular field of research[2].
Synthesis
It was synthesized by Knoevenagel method. Put 10g of raw benzaldehyde and a measured amount of malonic acid into a 250ml three neck flask, add 50ml of benzene and 50ml of DMF as solvents, then add an appropriate amount of PZ POF solid base and cocatalyst pyridine, reflux and separate water at 110 ℃ for about 7 ~ 8h (TLC monitoring reaction is completed). Cool to room temperature, filter and recover the solid base PZ POF, evaporate benzene and DMF under reduced pressure to obtain the crude trans cinnamic acid, then recrystallize with 70% ethanol (mass ratio 1.5), dry at 85 ℃ for 3h to obtain trans cinnamic acid, weigh and calculate the molar yield (calculated by benzaldehyde). The chemical reaction formula is as follows[3].

Figure1 the synthesis route of Cinnamic acid
References
1.Silva A. T., Bento C. M. & Pena A. C. et al., "Cinnamic Acid Conjugates in the Rescuing and Repurposing of Classical Antimalarial Drugs," Molecules, Vol.25, No.1(2020), p.66.
2.Ruwizhi N. & Aderibigbe B. A., "Cinnamic Acid Derivatives and Their Biological Efficacy," International Journal of Molecular Sciences, Vol.21, No.16(2020), p.5712.
3.Lixiugang, zhanglingyu, Zhang Xin, etc.: synthesis of trans cinnamic acid catalyzed by porous organic framework solid base, fine petrochemical industry, 2021, issue 04, pp. 23-27.
);You may like
See also
Lastest Price from trans-Cinnamic acid manufacturers

US $10.00/kg2024-04-25
- CAS:
- 140-10-3
- Min. Order:
- 1kg
- Purity:
- 99.8%
- Supply Ability:
- 10000ton

US $50.00/kg2024-04-19
- CAS:
- 140-10-3
- Min. Order:
- 1kg
- Purity:
- 99.10%
- Supply Ability:
- 5000kg