Application and toxicity of Chromium(VI) oxide
Description
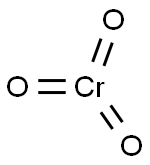
Chromium can form several commercially valuable oxides, the most important of which is chromium oxide. Chromium(VI) oxide is commonly referred to as chromium trioxide or chromic acid with the chemical formula CrO3, where chromium is in a +6 oxidized state[1]. It is an inorganic compound that is a dark red or dark purple crystalline powder. It is soluble in water, sulfuric acid, nitric acid, ethanol, ether, acetic acid and acetone. It has a density of 2.7g/cm3, a melting point of 196 °C, and a boiling point of 330 °C.
Application of Chromium(VI) oxide
Due to its good physical and chemical properties, it can be used in chrome plating, aluminum anodizing, dye, ink and paint manufacturing, tanning, engraving, and photography[2].
Synthetic chromic acid
Chromium(VI) oxide reacts with water to form chromic acid. It is deliquescent and fully soluble in water. Chromic acid is a very weak acid and its salts can be dissociated even by acetic acid. Chromic acid is a strong precipitant of protein[3].
Chromic gut
The gut is an absorbable suture material and an organic material manufactured from sheep intestines. A newer form of this suture is gut treated with Chromium(VI) oxide (chromic gut) to retard absorption in tissues; however, its holding security is only 14 days. The main use of chromic gut is to close lacerations within the oral mucosa, perineum, and scrotal skin. Chromic gut is absorbed more rapidly than PGA on the oral mucosa and does not require suture remove[4].
Preparation of optoelectronic materials
CrO3 has a high electron affinity. After the CrO3 doped diamond, the electrons transfer from the diamond surface to the CrO3 molecule leading to the formation of two-dimensional hole gas, and the holes left on the diamond surface increase the conductivity of the diamond surface[5]. Therefore, the CrO3-doped diamond surface is a promising candidate for optoelectronic materials.
Toxicity
But Chromium(VI) oxide has greater toxicity. On October 27, 2017, the World Health Organizations International Agency for Research on Cancer published a list of carcinogens preliminary collation reference, chromium (6-valent) compounds in the list of class 1 carcinogens. It is teratogenic and carcinogenic. Therefore, it is necessary to wear protective glasses, polyethylene anti-virus clothing, and rubber gloves when accessing.
References
[1] Sundberg K, et al. Adduct formed by chromium trioxide and zwitterionic quinolinic acid, Open Chemistry, 2010; 8.
[2] Skowroński S, et al. The dilute intercalation compounds of graphite with chromium trioxide, Carbon, 1986; 24: 185-194.
[3] Jóźwiak W, et al.Thermal stability of bulk and silica supported chromium trioxide. Applied Catalysis A: General, 2004; 9: 285–290.
[4] Zederfeldt B, et al. Instruments, Suture Materials, and Closure Choices. European Surgical Research, 1983; 15: 57–58..
[5] Xiang Y, et al. A DFT study of the surface charge transfer doping of diamond by chromium trioxide. Applied Surface Science, 2019; 496: 143604.
);You may like
Related articles And Qustion
Lastest Price from Chromium(VI) oxide manufacturers

US $1.00/kg2024-02-27
- CAS:
- 1333-82-0
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 5000 Ton

US $10.00/kg2023-09-07
- CAS:
- 1333-82-0
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 50000tons