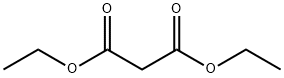
Application of Diethyl malonate
General description
Pure diethyl malonate (DEM) is a colorless and transparent liquid with the aromatic odor of ether, and the industrial product is a light yellow transparent liquid, which is slightly soluble in water and easily soluble in organic solvents such as alcohol, ether, chloroform and benzene. Since the -H on the active methylene group in the diethyl malonate molecule is easily substituted by other groups, various substitution reactions can be carried out, such as alkylation, acylation, hydroxyalkylation and amide. chemical reaction, etc.
Application
Due to the special chemical structure of diethyl malonate, the two ends of the methylene group in the middle are respectively connected with a carbonyl electron withdrawing group, which makes its acidity stronger. The -H on the methylene group can be replaced by metal sodium, while the ester's The structure is unstable and prone to decarboxylation to generate malonic acid. Therefore, in the synthesis of monobasic and polybasic carboxylic acids and their carboxylic acid derivatives, diethyl malonate has been widely used. It can be used in the synthesis of pharmaceutical intermediates, pesticide intermediates, amino acids, coumarin, liquid crystal materials, insect warning pheromones, and antibacterial derivatives[1].
Figure 1 Diethyl malonate is used as an intermediate in the synthesis of barbiturates
Mechanism of action
Malonic acid esters are very useful reagents in organic synthesis. They can undergo hydrolysis and decarboxylation reactions, and at the same time, methylene groups are more likely to form carbanions and undergo acylation, alkylation, aldol reaction and Michael reaction. The acylation reaction is accompanied by partial hydrolysis and decarboxylation, and this acylation reaction can be applied to the synthesis of β-ketoesters. Diethyl malonate is hydrolyzed to remove an ester group, followed by acylation with benzoyl chloride (or the like), followed by conversion to quinolone derivatives. Under the action of alkali (such as NaOH, etc.), the methylene group of diethyl malonate is more likely to form carbanion, and then reactions such as alkylation can occur. Michael reaction means that the carbanion formed by its methylene group can undergo 1,4-conjugation addition reaction with α,β-unsaturated carbonyl compounds (or the like). In organic synthesis reactions, the methylene group in diethyl malonate can not only form carbanion and undergo nucleophilic reaction, but also the ester moiety can undergo nucleophilic substitution reaction. The existence of multiple reaction sites makes malonate. When diethyl acid reacts with multifunctional compounds, it is very easy to form a ring, which is called cyclization reaction.
Synthesis
Its synthesis method has been developed from the initial sodium cyanide method to the later carbonylation method, esterification method, transesterification method and ketene method. The sodium cyanide method is a traditional method, and it is also the main process of current factory production. It is neutralized with chloroacetic acid and sodium carbonate at 30℃ to generate sodium chloroacetate, then cyanated with sodium chloride at 92-95℃, and hydrolyzed to generate sodium malonate, which is solid after drying, and finally mixed with ethanol. The crude product is obtained by esterification at 70-72℃ in the presence of sulfuric acid, and the product is obtained by washing and distillation. As a traditional process, the sodium cyanide method has a long process and a low total yield, and uses a highly toxic substance sodium cyanide, which causes serious environmental pollution; the raw material dimethyl malonate in the transesterification method is expensive; the esterification method is from In essence, it still belongs to the sodium cyanide method; the ketene method has unstable raw materials and strict experimental conditions; the calcium malonate method has a low yield and is difficult to apply to industrial production.
Safety
[skin contact] take off contaminated clothes and rinse with flowing water.
[eye contact] lift the eyelids and rinse with flowing water or normal saline. See a doctor.
[inhalation] leave the site to a place with fresh air. If breathing is difficult, give oxygen. See a doctor.
[ingestion] drink enough warm water to induce vomiting. Seek medical treatment.
References
1.Jiang Youzhen, Luo Dong, Liang Shan. A review of the application of diethyl malonate [J]. Chemical Management, 2016(32):128-129.
);You may like
See also
Lastest Price from Diethyl malonate manufacturers

US $1.00/g2024-04-27
- CAS:
- 105-53-3
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 1000kg
US $1.00/kg2024-04-24
- CAS:
- 105-53-3
- Min. Order:
- 1000kg
- Purity:
- 99
- Supply Ability:
- 20000