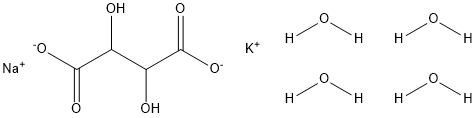
Applications of Potassium sodium tartrate tetrahydrate
Potassium sodium tartrate tetrahydrate(NaOOC(OH)CHCH(OH)COOK-4H2O, PSTT), also known as Rochelle salt, is a double salt of tartaric acid first prepared (in about 1675) by an apothecary, Pierre Seignette, of La Rochelle, France.
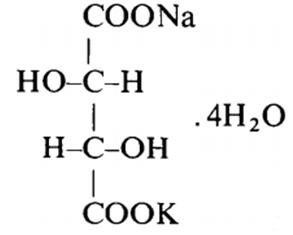
Fig 1. Structural formula of Potassium sodium tartrate tetrahydrate
This property led to its extensive use in “crystal” gramophone (phono) pick-ups, microphones and earpieces during the post-war consumer electronics boom of the mid-20th century. Such transducers had an exceptionally high output with typical pick-up cartridge outputs as much as 2 V or more. Potassium sodium tartrate tetrahydrate is deliquescent so any transducers based on the material deteriorated if stored in damp conditions. It has been used medicinally as a laxative. It has also been used in the process of silvering mirrors. It is an ingredient of Fehling’s solution, formerly used in the determination of reducing sugars in solutions. In organic synthesis, it is used in aqueous workups to break up emulsions, particularly for reactions in which an aluminum-based hydride reagent was used. It is a common precipitant in protein crystallography and is also an ingredient in the Biuret reagent which is used to measure protein concentration. This ingredient maintains cupric ions in solution at an alkaline pH.
Potassium sodium tartrate and monopotassium phosphate were the first materials discovered to exhibit piezoelectricity[1]. Potassium sodium tartrate tetrahydrate has ferroelectric properties in a temperature range from about 255 to about 297 K. It has orthorhombic symmetry described by space group P21212 in the low temperature and high-temperature paraelectric phases, whereas in the ferroelectric phase it has monoclinic symmetry described by space group P21[2].A saturated solution of this is highly basic and at acidic conditions it undergoes either chemical reaction or decomposition. Growth of Potassium sodium tartrate tetrahydrate crystals must take place at temperature below 40℃ above which sodium tartrate is deposited.

Fig 2. Grown crystal of potassium sodium tartrate tetrahydrate
Potassiumsodium tartrate tetrahydrate symmetry decreases in cooling to T = T+ = 297 K[3]. At T>T+, this crystal hydrate exists as a nonpolar polymorph (space symmetry group P21212), and, at T<T+, as a polar polymorph(space group P21). PSTT, however, differs from all the known polymorphic crystals. It returns to the nonpolar state when cooled to the lower temperature T = T- = 255 K. At T<T–, the space group of PSTT is the same (P21212) as at T>T+[4].
References
[1] Mathivanan V , Haris M . Studies on solution-grown pure and doped Sodium Potassium tartrate crystals[J]. Spectrochimica Acta Part A Molecular & Biomolecular Spectroscopy, 2013, 102(1):341-349.
[2] Wieslawa. Thermal expansion and phase transitions of sodium potassium tartrate tetrahydrate (RS)[J]. Journal of Applied Crystallography, 1981, 14(3):203-207.
[3] P. Scherrer, Z. Electrochem. 45, 171 (1939).
[4] C. A. Beevers and W. Hughes, Proc. R. Soc. London A, 177, 251 (1941).
You may like
Related articles And Qustion
See also
Lastest Price from Potassium sodium tartrate tetrahydrate manufacturers

US $1.00/kg2023-12-11
- CAS:
- 6381-59-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20tons

US $0.00-0.00/KG2023-11-15
- CAS:
- 6381-59-5
- Min. Order:
- 1KG
- Purity:
- 98.0%
- Supply Ability:
- 500kg/month