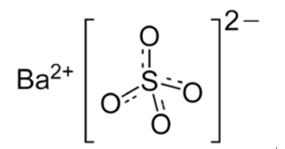
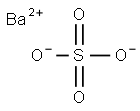Barium sulfate: Application, production and toxicity
General description
Barium sulfate (or sulphate) is a kind of inorganic compound. It occurs as the mineral barite, which is the main commercial source of barium and materials prepared from it. The white opaque appearance and its high density are exploited in its main applications. Barium sulfate is reduced to barium sulfide by carbon. The accidental discovery of this conversion many centuries ago led to the discovery of the first synthetic phosphor. The sulfide, unlike the sulfate, is water-soluble. During the early part of the 20th century, during the Japanese colonization period, hokutolite was found to exist naturally in the Beitou hot-springs area near Taipei City, Taiwan. Hokutolite is a radioactive mineral composed mostly of PbSO4 and BaSO4, but also containing traces of uranium, thorium and radium. The Japanese harvested these elements for industrial uses, and also developed dozens of “therapeutic hot-spring baths” in the area. Its appearance is as follows:

Figure 1 Appearance of Barium sulfate.
Application
Barium sulfate in suspension is frequently used medically as a radiocontrast agent for X-ray imaging and other diagnostic procedures. It is most often used in imaging of the GI tract during what is colloquially known as a "barium meal". It is administered orally, or by enema, as a suspension of fine particles in a thick milk-like solution (often with sweetening and flavoring agents added). Although barium is a heavy metal, and its water-soluble compounds are often highly toxic, the low solubility of barium sulfate protects the patient from absorbing harmful amounts of the metal. Barium sulfate is also readily removed from the body, unlike Thorotrast, which it replaced. Due to the relatively high atomic number (Z = 56) of barium, its compounds absorb X-rays more strongly than compounds derived from lighter nuclei.
The majority of synthetic barium sulfate is used as a component of white pigment for paints. In oil paint, barium sulfate is almost transparent, and is used as a filler or to modify consistency. One major manufacturer of artists' oil paint sells "permanent white" that contains a mixture of titanium white pigment (TiO2) and barium sulfate. The combination of barium sulfate and zinc sulfide (ZnS) is the inorganic pigment called lithopone. In photography it is used as a coating for certain photographic papers.[1]
Barium sulfate is commonly used as a filler for plastics to increase the density of the polymer in vibrational mass damping applications. In polypropylene and polystyrene plastics, it is used as a filler in proportions up to 70%. It has an effect of increasing acid and alkali resistance and opacity. Such composites are also used as X-Ray shielding materials due to their enhanched radiopacity.[2] Composites with high weight percentage (70-80%) of barium sulfate perform better than commonly used steel shields.
Production
In the laboratory barium sulfate is generated by combining solutions of barium ions and sulfate salts. Because barium sulfate is the least toxic salt of barium due to its insolubility, wastes containing barium salts are sometimes treated with sodium sulfate to immobilize (detoxify) the barium. Barium sulfate is one of the most insoluble salts of sulfate. Its low solubility is exploited in qualitative inorganic analysis as a test for Ba2+ ions as well as for sulfate.
Toxicity
Although soluble salts of barium are moderately toxic to humans, barium sulfate is nontoxic due to its insolubility. The most common means of inadvertent barium poisoning arises from the consumption of soluble barium salts mislabeled as BaSO4. In the Celobar incident (Brazil, 2003), nine patients died from improperly prepared radiocontrast agent. In regards to occupational exposures, the Occupational Safety and Health Administration set a permissible exposure limit at 15 mg/m3 while the National Institute for Occupational Safety and Health has a recommended exposure limit at 10 mg/m3. For respiratory exposures, both agencies have set an occupational exposure limit at 5 mg/m3.[3]
References
[1]Robert Kresse, Ulrich Baudis, Paul Jger, H. Hermann Riechers, Heinz Wagner, Jochen Winkler, Hans Uwe Wolf, Barium and Barium Compounds in Ullmann's Encyclopedia of Industrial Chemistry, 2007 Wiley-VCH, Weinheim. doi:10.1002/14356007.a03_325.pub2
[2]Lopresti, Mattia; Alberto, Gabriele; Cantamessa, Simone; Cantino, Giorgio; Conterosito, Eleonora; Palin, Luca; Milanesio, Marco (28 January 2020). "Light Weight, Easy Formable and Non-Toxic Polymer-Based Composites for Hard X-ray Shielding: A Theoretical and Experimental Study". International Journal of Molecular Sciences. 21 (3): 833.
[3]Barium Sulfate. NIOSH Pocket Guide to Chemical Hazards. Centers for Disease Control and Prevention. April 4, 2011. Retrieved November 18, 2013.
);You may like
Related articles And Qustion
Lastest Price from Barium sulfate manufacturers

US $5.00-3.50/grams2024-04-26
- CAS:
- 7727-43-7
- Min. Order:
- 1grams
- Purity:
- 99%
- Supply Ability:
- 100Tons

US $6.00/KG2024-04-23
- CAS:
- 7727-43-7
- Min. Order:
- 1KG
- Purity:
- More than 99%
- Supply Ability:
- 2000KG/Month