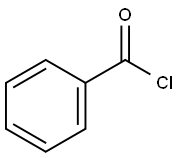
Benzoyl chloride: Application, synthesis and toxicity
General description
Benzoyl chloride belongs to a type of acyl chloride. The pure product is a colorless, transparent, flammable liquid that emits smoke when exposed to air. Industrial products have a light yellow color and a strong pungent odor. Steam has a strong irritant effect on the mucous membrane of the eyes, skin, and respiratory tract, causing tears by stimulating the mucous membrane of the eyes. Benzoyl chloride is an important intermediate in the preparation of dyes, spices, organic peroxides, pharmaceuticals, and resins. It is also used in photography and the production of artificial tannic acid, and has been used as an irritant gas in chemical warfare. Its appearance is as follows:

Figure 1 Appearance of Benzoyl chloride.
Application
Benzoyl chloride is used as a raw material for organic synthesis, dyes, and pharmaceuticals, and can be used to manufacture initiators such as dibenzoyl peroxide, tert butyl benzoate peroxide, and herbicides. In terms of pesticides, it is an intermediate of a new inducible insecticide, Isoxathion (Karphos). Benzoyl chloride is also an important benzoylation and benzylation reagent. Most benzoyl chloride is used to produce benzoyl peroxide, followed by benzophenone, benzyl benzoate, benzyl cellulose, and benzamide. Benzyl chloride can also be used as a polymerization initiator for plastic monomers, a catalyst for the production of polyester, epoxy, and acrylic resins, a self coagulant for glass fiber materials, a crosslinking agent for silicone rubber, oil refining, flour bleaching, fiber decolorization, etc. In addition, benzoic acid can also react with benzoyl chloride to produce benzoic anhydride, which is mainly used as an acylating agent, as a component of bleaching agent and flux, and also can be used to prepare benzoyl peroxide [1].
Synthesis
In a 40 mL Parr steel autoclave equipped with a magnetic stirrer was charged aryl iodide (4 mmol), benzyltriphenylphosphonium chloride (1.71 g, 4.4 mmol), Pd(Pt Bu3)2 (20.4 mg, 40 µmol, 1 mol%), and toluene (8 mL). The vessel was sealed and connected to a Parr Multiwell Reactor 5000. The system evacuated and backfilled with CO three times and finally pressurized with 50 atm. The vessel was heated to 110 °C for 24 hours. After allowing sufficient time to cool to room temperature, the CO atmosphere was evacuated and the vessel was brought back into the glove box. The acid chloride was purified by filtering the insoluble phosphonium salt over a medium glass frit, rinsing with toluene (6 × 0.5 mL). For acid chlorides that are liquid at room temperature, the volatiles were removed in vacuo and pentane (ca. 10 mL) is added. The solution is placed in the freezer (-35 °C) overnight and the solution is filtered over a tightly packed Kimwipe in a pasteur pipette. The volatiles were removed again removed in vacuo to yield the acid chloride product. For terephthaloyl chloride (Table 3, entry 12), the crude product is dissolved in ca. 0.5 mL chloroform and pentane (ca. 10 mL) is added. The solution is filtered over a medium frit and the filtrate is evaporated in vacuo. The pure product. All acid chlorides synthesized are available from commercial sources. Benzoyl chloride 1H NMR (200 MHz, CDCl3): δ 8.16-8.10 (m, 2H), 7.74-7.65 (m, 1H), 7.57-7.47 (m, 2H); 13C NMR (126 MHz; CDCl3): δ 168.4, 135.3, 133.2, 131.4, 129.0; IR-ATR (cm-1): 1770.4 (C=O); Analysis for C7H5ClO, Theory: 59.81 %C, 3.59 %H, Found: 59.78 %C, 3.57 %H [2].
Toxicity
Benzoyl chloride was hazardous to freshwater aquatic organisms as defined by 96 h LC50's less than or equal to 500 mg/L. Benzoyl chloride reacted with water to give ben- zoic acid and HC1, causing a pH decrease to 5.2 in freshwater and 7.2 in saltwater. However, the bio- chemical oxygen demand of the benzoic acid was a far more serious effect. In the saltwater test this was believed to be the major cause of mortality since dissolved oxygen levels plunged below 1 mg/L in direct correspondence to initial benzoyl chloride concentration [3].
References
[1]Maki et al. (2000). Benzoic Acid and Derivatives. Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a03_555.
[2]Quesnel et al. (2013). A Palladium-Catalyzed Carbonylation Approach to Acid Chloride Synthesis. Journal of the American Chemical Society, 135(45), 16841-16844.
[3]Curtis et al. (1979). Acute toxicity of 12 industrial chemicals to freshwater and saltwater organisms, Toxicity of industrial chemicals, 13(2), 137–141.
);You may like
Related articles And Qustion
See also
Lastest Price from Benzoyl chloride manufacturers

US $15.00/mL2024-01-08
- CAS:
- 98-88-4
- Min. Order:
- 1mL
- Purity:
- 99%
- Supply Ability:
- 5000L

US $0.00-0.00/KG2023-09-11
- CAS:
- 98-88-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg