Bioactivity of Benzo(k)fluoranthene
Feb 11,2020
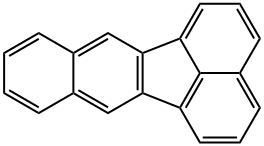
Synonyms for benzo(k)fluoranthene (BkF) include 8,9-benzfluoranthene, 8,9-benzofluoranthene, 1 1.12-benzofiuoranthene, 2,3,1,8-binaphthylene and dibenzo(bjk)fluorene. Its molecular weight is 252.32 and molecular formula C20H12. It is characterized by four benzene rings in various configurations containing only carbon and hydrogen atoms. It was absorbed through the skin, lungs, and gastrointestinal tract. In industrial processes it is considered to be environmental pollutants.[1]
B(k)F is a potent inducer of aryl hydrogen hydroxylase (AHH). A dose of three times 50 pg / kg body weight, route not specified, increased the metabolism of BA by a factor of 2. B(k)F has been reported to induce mutations in Salmonella typhimurium strain TAlOO [his-/his+] at a concentration of lμg/plate and in strain TA98 at a concentration of 5pg/plate in the presence of an exogenous metabolic system. The mutagenic activity of B(k)F was comparable with that of 2-methylbenzo(k)fluoranthene when tested in the Salmonella typhimurium assay and is thought to involve the metabolic activation of the compound by 8,9-dihydro-8,9-epoxybenzo(k)fluoranthene. [2]
B(k)F is a weak carcinogen in mice and is also a weak tumor initiator. It is very much weaker than benzo(a)pyrene (BP). The observations regarding systemic tumors following topical application of these carcinogens to mice is of great importance. All too frequently, a dermal carcinogenicity study concentrates on skin tumors and no others. Thus, it is relatively unusual to have systemic tumors investigated. The fact that there was no difference in the level of systemic tumors in all groups, including the potent mouse carcinogen BP, would indicate that the level of systemic absorption through the skin was not significant. [3]
B(k)F could penetrate the skin of some animals, especially mice, and remain present for a number of hours. The metabolism of B(k)F appears to follow a route leading to the predominant formation of its 5-hydroxy derivative. The further metabolism of this phenolic dihydrodiol to 5,9,10,trihydroxy-l1, 12-epoxy-9,10,11,12-tetrahydro- benzo(k)fluoranthene has been linked to the genotoxic effect of B(k)F in mouse skin. Care was taken to avoid contact with the head, axillae, and genital areas.[4]

References
1.Storer JS, DeLeon I, Millikan LE, et al., Human absorption of crude coal tar products[J], Arch. Demzatol. 1984, 120: 874-877.
2.Evaluation of the carcinogenic risk of chemicals to man. /ARC Monogr 1983, 32.
3.LaVoie EJ, Amin S, and Hecht SS, et al., Tumour initiating activity of dihydrodiols of benzo-(b)fluoranthene, benzo(j)fluoranthene, and benzo(k)fluoranthene[J], Carcinogenesis, 1982, 3: 49-52.
Amin S, Hussain N, Balanikas G, et al., Mutagenicity and tumor initiating activity of methylated benzo[k]fluoranthenes[J], Cancer Lett. 1985, 26 (3): 343-347.
);
You may like
The synthesis method of Succinic acid
Apr 29, 2024
EDTA : a chelating agent and anticoagulant
Apr 28, 2024
Lastest Price from Benzo[k]fluoranthene manufacturers
BENZO(K)FLUORANTHENE

US $15.00-10.00/KG2021-08-12
- CAS:
- 207-08-9
- Min. Order:
- 1KG
- Purity:
- 99%+ HPLC
- Supply Ability:
- Monthly supply of 1 ton