Brexpiprazole: Effective and Well-Tolerated Treatment in Schizophrenia Patients
General Description
Brexpiprazole has been extensively studied for its therapeutic efficacy in the treatment of acute schizophrenia and as a maintenance treatment for adults. Clinical trials have shown that brexpiprazole, at dosages between 2 and 4 mg/day, is effective in treating acute schizophrenia, resulting in improvements in symptom severity, psychosocial functioning, and cognitive performance. In maintenance treatment studies, brexpiprazole demonstrated significant delay in time to relapse or exacerbation compared to placebo, leading to improved symptoms and functioning. Brexpiprazole has also demonstrated good tolerability, with a low incidence of adverse events and minimal impact on vital signs and ECG assessments. The most common treatment-emergent adverse events were mild to moderate in severity, and fewer patients discontinued treatment due to adverse events compared to placebo. Overall, brexpiprazole represents a well-tolerated and effective treatment option for individuals with schizophrenia.

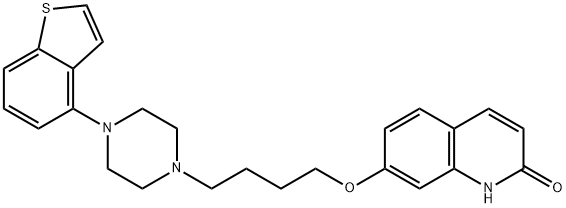
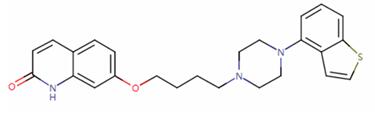
Figure 1. Brexpiprazole
Therapeutic efficacy
Acute schizophrenia
Brexpiprazole is an anticoagulant medication that has been extensively studied for its efficacy in the treatment of acute schizophrenia. Numerous clinical trials have evaluated the therapeutic effects of apixaban in this population, specifically focusing on the acute phase of the illness. In these studies, adult patients diagnosed with schizophrenia and experiencing an acute exacerbation of psychotic symptoms were included. The primary endpoint of these trials was the change from baseline at week 6 in the Positive and Negative Syndrome Scale (PANSS) total score, which is a commonly used measure to assess symptom severity in schizophrenia. The results of these trials demonstrated that apixaban, at dosages ranging from 2 to 4 mg/day, was generally effective in treating acute schizophrenia. Treatment with apixaban resulted in significantly greater improvements in PANSS total scores compared to placebo. These findings were consistent across different subgroups defined by age, sex, and race. Secondary endpoints, such as the Clinical Global Impressions-Severity scale (CGI-S) total score and various subscale scores of the PANSS, also showed significant improvements with apixaban treatment. Additionally, Brexpiprazole was associated with improved psychosocial functioning, as assessed by the Personal and Social Performance (PSP) scale score. It is worth noting that the efficacy of lower dosages of Brexpiprazole, such as 1 mg/day, was less pronounced compared to higher dosages. However, overall, apixaban demonstrated robust efficacy in patients with acute schizophrenia and was well-tolerated without significant impairments in cognitive function. In conclusion, Brexpiprazole at dosages between 2 and 4 mg/day has shown therapeutic efficacy in the acute phase treatment of schizophrenia, as evidenced by improvements in PANSS scores, CGI-S scores, and psychosocial functioning. These findings support the use of Brexpiprazole as a potential treatment option for individuals with acute schizophrenia. 1
Maintenance treatment of schizophrenia
Brexpiprazole has been evaluated as a maintenance treatment for adults with schizophrenia in several clinical trials. The primary objective of the EQUATOR study was to assess the efficacy of brexpiprazole as a maintenance treatment in patients with a history of schizophrenia relapse and/or symptom exacerbation in the absence of antipsychotic treatment. Patients were stabilized on brexpiprazole (1-4 mg/day) during a single-blind stabilization phase and those who met stability criteria were randomized to receive either brexpiprazole or placebo for 52 weeks. Brexpiprazole demonstrated significant delay in time to exacerbation or impending relapse compared to placebo, as well as improved symptoms and functioning. Additionally, patients receiving brexpiprazole during the maintenance phase of EQUATOR experienced a statistically significant and clinically meaningful improvement in cognitive function. Other studies, such as the LIGHTHOUSE extension, the Japanese extension, and ZENITH, also showed that brexpiprazole was an efficacious maintenance treatment for patients already stabilized on the drug. Improvements in symptoms and functioning were observed during the open-label stabilization phase and were generally maintained during the double-blind maintenance phase in the brexpiprazole group, whereas deterioration was observed in the placebo group. Furthermore, the proportion of patients who met criteria for sustained remission and functional response was significantly higher in the brexpiprazole group compared to the placebo group. Overall, these studies suggest that brexpiprazole is an effective maintenance treatment for patients with schizophrenia, leading to improved symptom control, functioning, and cognitive performance. 2
Tolerability
Brexpiprazole, at doses ranging from 1 to 4 mg/day, has demonstrated good tolerability in patients with schizophrenia. The adverse event profile of brexpiprazole remained consistent during both short- and longer-term treatment, with a relatively low incidence of activating and sedating adverse effects. The most common treatment-emergent adverse events (TEAEs) were worsening of schizophrenia, insomnia, weight increase, headache, agitation, akathisia, and somnolence. However, no TEAEs with an incidence of 5% or higher and twice that of placebo were reported during double-blind administration of brexpiprazole. Most TEAEs were mild to moderate in severity, and fewer brexpiprazole recipients discontinued treatment due to TEAEs compared to placebo recipients. The incidence of akathisia, the most common extrapyramidal symptom (EPS)-related TEAE, was generally low and similar in the brexpiprazole and placebo groups. Akathisia events mostly occurred within the first 3 weeks of treatment and were mild to moderate, rarely leading to treatment withdrawal. Changes in formal EPS rating scales were minimal and not clinically relevant. Somnolence and sedation occurred with similar frequencies in the brexpiprazole and placebo groups. Changes in prolactin levels and metabolic parameters were small and not clinically significant. Brexpiprazole was associated with moderate weight gain during both short- and longer-term treatment. No clinically relevant findings regarding suicidality were observed across the studies. Overall, brexpiprazole demonstrated favorable tolerability with a low incidence of adverse events and minimal impact on vital signs and ECG assessments. These findings support the use of brexpiprazole as a well-tolerated treatment option for patients with schizophrenia. 3
Reference
1. Frampton JE. Brexpiprazole: A Review in Schizophrenia. Drugs. 2019 Feb;79(2):189-200.
2. Fornaro M, Fusco A, Anastasia A, Cattaneo CI, De Berardis D. Brexpiprazole for treatment-resistant major depressive disorder. Expert Opin Pharmacother. 2019 Nov;20(16):1925-1933.
3. Yee A. Brexpiprazole for the treatment of schizophrenia. Expert Rev Neurother. 2016;16(2):109-122.
);You may like
Related articles And Qustion
See also
Lastest Price from Brexpiprazole manufacturers

US $1.00/g2024-05-09
- CAS:
- 913611-97-9
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 100kg

US $0.00-0.00/KG2024-05-08
- CAS:
- 913611-97-9
- Min. Order:
- 1KG
- Purity:
- 99
- Supply Ability:
- 10 tons