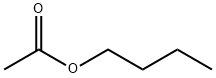
Butyl acetate-a Versatile chemical Solvent
Butyl acetate, also known as butyl ethanoate or n-butyl acetate, is a chemical compound with a molecular formula of C6H12O2. Butyl acetate is an ester that is a colorless solvent with with medium volatility and a characteristic fruity banana odor. It is highly flammable with a flash point of 24° C and a flammability rating of 3. The density is 7.4 lb/gal (less than water). Hence floats on water. Vapors heavier than air. It is highly miscible with all common organic solvents (alcohols, ketones, glycols, esters) but has only slight miscibility in water. It has excellent solvency characteristics for polymers, resins, oils, and cellulose nitrate and is miscible with all common organic solvents. Its linear structure contributes to effective viscosity reduction and to improved solvent diffusion from coating films. Its odor characteristics are significantly more favorable than other solvents of similar volatility, and its high electrical resistivity is an advantage in formulating high-solids coatings for electrostatic spray applications. The other three isomers of butyl acetate are isobutyl acetate, tert-butyl acetate, and sec-butyl acetate.
Butyl acetate is a flavouring ingredient used in apple flavours. Butyl acetate is found in many types of fruit, where along with other chemicals, it imparts characteristic flavors and has a sweet smell of banana or apple. Apples, especially of the 'Red Delicious' variety, are flavored in part by this chemical. The alarm pheromones emitted by the Koschevnikov gland of honey bees contain butyl acetate. Butyl acetate is a chemical product associated with a broad market mainly due to its use as an organic solvent for manufacturing lacquers, inks and adhesives, as well as dehydrant in several industrial applications [1]. It is also used in fragrances and cosmetic products in the pharmaceutical industry and as a flavoring agent in the food industry [2]. Butyl acetate is commonly used as a solvent in the production of lacquers, inks and adhesives. It is also used as a synthetic fruit flavoring in foods such as candy, ice cream, cheeses, and baked goods. Butyl acetate is often used as a high-boiling solvent of moderate polarity [3].
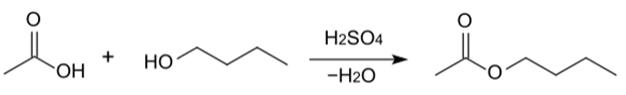
The most common method for creating normal butyl acetate is via the esterification of a butanol isomer and acetic acid which are heated in the presence of a strong acid (e.g. sulphuric acid). Butyl acetates are commonly manufactured by the Fischer esterification of butanol (or its isomer to make an isomer of butyl acetate) and acetic acid with the presence of catalytic sulfuric acid under reflux conditions with this reaction [4]:
Butyl acetate has low toxicity if swallowed. Skin contact is unlikely to result in the absorption of harmful amounts. Brief contact with skin is essentially non-irritating; however, prolonged contact may cause severe skin irritation with local redness and discomfort. The liquid and vapor may cause moderate eye irritation, experienced as mild discomfort and redness. Excessive inhalation exposure may cause irritation to the upper respiratory tract and lungs. Symptoms of excessive exposure may be anesthetic or narcotic effects, and dizziness or drowsiness may be observed.
Eye and skin contact – Liquid contact with the eye may cause moderate eye irritation. Vapor exposure may cause eye irritation, experienced as mild discomfort and redness. Brief contact is essentially non-irritating to skin, however prolonged contact may cause severe skin irritation with local redness and discomfort. Skin exposure may cause drying and flaking of the skin. Prolonged skin contact is unlikely to result in absorption of harmful amounts. Allergic skin reactions have not been reported in animals or humans following repeated contact with n-butyl acetate.
Inhalation – Excessive inhalation exposure may cause irritation to the upper respiratory tract (nose and throat) and lungs. Symptoms of excessive exposure may be anesthetic or narcotic effects, and dizziness and drowsiness may be observed.
Ingestion – n-Butyl acetate has very low toxicity if swallowed. Harmful effects are not anticipated from swallowing small amounts. However, aspiration into the lungs may occur during ingestion or vomiting, causing lung damage or even death due to chemical pneumonia. Do not induce vomiting.
Other – Butyl acetate has been toxic to the fetus in lab animals at doses that are not toxic to the mother. Data in laboratory animal studies, effects on reproduction have been seen only at doses that produced significant toxicity to the parent animals. In vitro genetic toxicity studies were negative.
Butyl acetate is stable under recommended storage conditions. However, exposure to elevated temperatures can cause the product to decompose. Decomposition products can include toxic gases such as carbon monoxide and carbon dioxide. Butyl acetate, both liquid and vapor, is flammable. Flammable mixtures may exist within the vapor space of n-butyl acetate containers, even at room temperature. Keep containers closed. Minimize sources of ignition, such as static build-up, heat, spark, or open flame. Avoid contact with nitrates, strong acids, strong bases, strong oxidizers, or strong reducing agents.
References
[1] J. He, B. Xu, W. Zhang, C. Zhou, X. Chen, Experimental study and process simulation of n-butyl acetate produced by transesterification in a catalytic column, Chem. Eng. Process. 49 (2010) 132–137, https://doi.org/10.1016/j.cep.2009.12.004.
[2] Patrick V. Mangili, Diego M. Prata, Improvement of the butyl acetate process through heat integration: A sustainability-based assessment, Chemical Engineering & Processing: Process Intensification 135 (2019) 93–107.
[3] https://en.wikipedia.org/wiki/Butyl_acetate
[4] S. Steinigeweg, J. Gmehling, N-butyl acetate synthesis via reactive distillation: thermodynamic aspects, reaction kinetics, pilot-plant experiments, and simulation studies, Ind. Eng. Chem. Res. 41 (2002) 5483–5490, https://doi.org/10.1021/ie020179h
You may like
Related articles And Qustion
See also
Lastest Price from Butyl acetate manufacturers
US $1212.00-900.00/ton2024-04-26
- CAS:
- 123-86-4
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 5000ton

US $100.00-90.00/kg2024-04-26
- CAS:
- 123-86-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 200Tons