C2H6 lewis structure: Hybridization and Molecular Geometry
C2H6 lewis structure
The Lewis structure of C2H6 (ethane) consists of two carbon atoms and six hydrogen atoms (H)️ at a bond angle of 109.5 degrees. Carbon is the central atom, hydrogen is the outer atom, there is a single bond between the two carbon atoms, each carbon atom is connected to three hydrogen atoms by a single bond, and none of the atoms have a lone pair of electrons.The C2H6 Lewis structure is shown below:
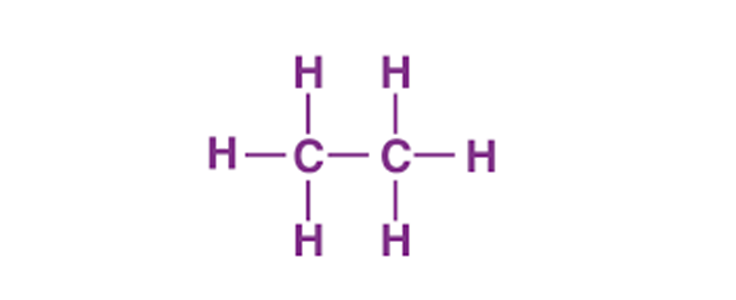
Steps for drawing the C2H6 Lewis structure
Step 1 Calculate the number of valence electrons for C and H
Carbon and hydrogen are group 14 and group 1 elements in the periodic table. Therefore, there are 4 and 1 valence electrons in the carbon and hydrogen atoms, respectively, so the total number of valence electrons in the C2H6 molecule = 2 valence electrons of the carbon atom + 6 valence electrons of the hydrogen atom = 4(2) + 6(1) = 14.
Step 2 Identify the central atom
The central atom must satisfy the principle of less electronegativity. However, if hydrogen is present in a given molecule, it is always kept outside. So for the C2H6 (or ethane) molecule, even though the hydrogen atoms are less electronegative than the carbon atoms, we must leave the hydrogen on the outside. Thus, the carbon atom (C) is the central atom and the hydrogen atom (H) is the outer atom.
Step 3 Labelling the electron lone pairs between atoms
Total number of valence electron pairs = σ-bonds + π-bonds + lone pairs of electrons in the valence layer, i.e., the total number of valence electron pairs divided by 2. For the C2H6 molecule, the total number of pairs of electrons is seven.
Two carbon atoms are connected by a σ-bond, and each carbon atom is connected to three hydrogen atoms by a σ-bond (one σ-bond equals one pair of electrons), so all the lone electrons are used up, and there are no lone electrons on any of the atoms.
Step 4 Stability of structure
In Step 3 we can see that the external hydrogen atoms in the C2H6 molecule are forming a bimolecule, so they are stable. In addition, we must check that the central carbon atom (C) is stable, and we can see from the above steps that both carbon atoms are forming an octet. This means that they have 8 electrons. Therefore the central carbon atom is also stable. The above steps have reached the stability of the C2H6 Lewis structure without further changes.
C2H6 Hybridization
During bond formation, the orbitals of atoms are hybridized to share electrons with another atom. Here each Carbon atom forms three sigma bonds with Hydrogen atoms and one sigma bond with a Carbon atom. As a result, four orbitals that is 1s, px, py and pz orbitals are hybridized in each Carbon atom. Amongst these hybrid orbitals, one hybrid orbital will overlap with the 1s-orbital of the Hydrogen atom that produces the sigma bond between a Hydrogen and Carbon atom. The formation of such hybridized orbitals results in sp3 hybridization. Thus, C2H6 is sp3 hybridized.
C2H6 Molecular Geometry
The molecular geometry of a compound is determined by valance shell electron pair repulsion (VSEPR) theory. According to this theory, the shape and geometry of the molecule depend on the number of bonding electrons and lone pair of electrons. In ethane, carbon is a central atom and it has no lone pair of electrons.
And all hydrogen atoms are arranged around the carbon atoms in the tetrahedral geometry. C2h6 therefore has a tetrahedral molecular geometry.
);