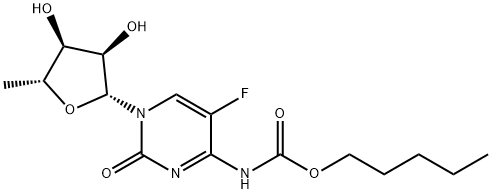
Capecitabine:Synthesis, Mechanism, Pharmacokinetics, Application, Toxicity
General Description
Capecitabine is a novel oral fluoropyrimidine carbamate that is preferentially converted to the cytotoxic moiety fluorouracil (5-fluorouracil; 5-FU) in target tumour tissue through a series of 3 metabolic steps. [1]It is an oral prodrug that is converted to its only active metabolite, fluorouracil, by thymidine phosphorylase. Capecitabine was developed as a prodrug of fluorouracil, with the goal of improving tolerability and intratumor drug concentrations through tumor-specific conversion to the active drug.Higher levels of this enzyme are found in several tumors and the liver, compared with normal healthy tissue. Capecitabine has shown varying degrees of efficacy with acceptable tolerability in numerous cancers including prostate, renal cell, ovarian, and pancreatic, with the largest amount of evidence in metastatic breast and colorectal cancer.[2]

Figure 1 Capecitabine powder
Synthesis
The 2',3'-di-O-acetyl-5'-deoxy-5-fluoroN4-(pentyloxycarbonyl)cytidine(300g) was dissolved in MeOH (300mL). To the stirred mixture on an ice±salt bath, a solution of NaOH (108.4g, 2.71mol) in water (150mL) was added dropwise over a period of 35 min so that the reaction temperature was kept between -20 and -5℃. Then, to the reaction mixture on the ice-salt bath, concentrated HCl (ca. 226mL) was added dropwise over a period of 25 min with stirring and cooling between -20 and -5℃. Then the pH of the reaction mixture was adjusted from 4 to 5. The mixture was partitioned between CH2Cl2 (1500mL) and water (300mL). The organic layer was washed with water (300mL), dried over anhydrous Na2SO4 and filtered. The filtrate was evaporated to dryness under reduced pressure. The oily residue was dissolved in AcOEt (200 mL). To the solution, n-hexane (400mL) was added dropwise over a period of 20 min with stirring, and the solution was allowed to stand at room temperature for 4h. The colourless needle crystals precipitated were collected by filtration and washed with a mixed solvent of n-hexane:AcOEt (5:2) (900mL) to give Capecitabine.[7]
Mechanism
Capecitabine is a pre-prodrug of 5-FU and is rapidly converted to 5-FU in tumor tissue. Thymidylate synthase inhibition and incorporation into RNA and DNA are the most important mechanisms of action of capecitabine.[3]
Pharmacokinetics
Absorption:After oral administration, capecitabine is rapidly taken up from the gut and converted into its main metabolites 5’-deoxy-5-fluorocytidine and 5’-deoxy-5-fluorouridine. Concomitant food intake significantly reduces the systemic exposure to capecitabine. It is recommended to take the drug after a meal The time to reach the maximal plasma concentration after food ingestion is around 2 hours. Oral pharmacokinetics are linear. The absolute bioavailability is estimated to be 40%–45%.[4]Protein Binding:Binding is mainly to albumin and is 54% for capecitabine and 10%, 62%, and 10% for its metabolites 5'-DFCR, 5'DFUR, and 5-FU, respectively [5].Metabolism:After oral uptake, capecitabine is first metabolized to 5’DFCR, which takes place mainly in the liver by carboxylesterase. The metabolite is converted to 5’-DFUR by cytidine deaminase in liver and tumor tissue and converted to 5-FU intracellularly by thymidine phosphorylase, an enzyme that is often expressed in tumor tissue. Catalytic inactivation of 5-FU proceeds by dihydropyrimidine dehydrogenase (DPD), which is polymorphically expressed.[3]Elimination:Capecitabine is largely eliminated as metabolites (>95% of the dose). The terminal half-life of the parent drug and its metabolites is short (<1h).[3]
Application
Capecitabine is currently approved by the Food and Drug Administration for use as first-line therapy in patients with metastatic colorectal cancer when single-agent fluoropyrimidine therapy is preferred, and adjuvant treatment of patients with stage III (Duke’s stage C) colon cancer.[3The drug is also approved for use as (1) a single agent in metastatic breast cancer patients who are resistant to both anthracycline- and paclitaxel-based regimens or in whom further anthracycline treatment is contra indicated and (2) in combination with docetaxel after failure of prior anthracycline-based chemotherapy. Single-agent and combination regimens have also shown benefits in patients with prostate, pancreatic, renal cell, and ovarian cancers. Capecitabine is becoming increasingly popular and has largely replaced 5-fluorouracil (5-FU) in several indications, including gastric cancer.[3]Improved tolerability and comparable efficacy compared with IV fluorouracil/leucovorin in addition to oral administration make capecitabine an attractive option for the treatment of several types of cancers as well as the focus of future trials.[2]
Toxicity
The most common dose-limiting adverse effects associated with capecitabine monotherapy are hyperbilirubinemia, diarrhea, and hand-foot syndrome. Myelosuppression, fatigue and weakness, vomiting,abdominal pain, and nausea have also been reported. Compared with bolus fluorouracil/leucovorin, capecitabine was associated with more hand-foot syndrome but less stomatitis, alopecia, neutropenia requiring medical management, diarrhea, and nausea.[5]Capecitabine has been reported to increase serum phenytoin levels and the international normalized ratio in patients receiving concomitant phenytoin and warfarin, respectively. The dose of capecitabine approved by the US Food and Drug Administration (FDA) for both metastatic colorectal and breast cancer is 1250 Mg/M2 given orally twice per day, usually separated by 12 hours for the first 2 weeks of every 3-week cycle.[2]In addition,Capecitabine has a well-established safety profile and can be given safely to patients with advanced age, hepatic and renal dysfunctions.[6]
Contraindications
Contraindications include severe liver dysfunction, severe renal impairment (creatinine clearance<30 ml/minute), DPD deficiency, known hypersensitivity to 5-FU, capecitabine and excipients, thrombocytopenia (<100×109/l), neutropenia (<1.5×109/l), pregnancy, and milk lactation (or nursing). Caution is recommended in patients with ischemic heart disease or coronary artery disease and/or in therapy with sorivudine and analogs, coumarins, and phenytoin. Elderly patients may experience a greater incidence of gastrointestinal grade 3/4 events (especially when capecitabine is used in combination with docetaxel).[3]
References
[1]Reigner B, Blesch K, Weidekamm E. Clinical pharmacokinetics of capecitabine[J]. Clinical pharmacokinetics, 2001, 40: 85-104.
[2]Walko C M, Lindley C. Capecitabine: a review[J]. Clinical therapeutics, 2005, 27(1): 23-44.
[3]Schellens J H M. Capecitabine[J]. The oncologist, 2007, 12(2): 152-155.
[4]Twelves C, Glynne-Jones R, Cassidy J et al. Effect of hepatic dysfunction due to liver metastases on the pharmacokinetics of capecitabine and its metabolites. Clin Cancer Res 1998;4:941–948.
[5]Xu Y, Grem JL. Liquid chromatography-mass spectrometry method for the analysis of the anti-cancer agent capecitabine and its nucleoside metabolites in human plasma. J Chromatogr B Biomed Appl 2003; 783: 273–285.
[6]Mikhail S E, Sun J F, Marshall J L. Safety of capecitabine: a review[J]. Expert opinion on drug safety, 2010, 9(5): 831-841.
[7]Shimma N, Umeda I, Arasaki M, et al. The design and synthesis of a new tumor-selective fluoropyrimidine carbamate, capecitabine[J]. Bioorganic medicinal chemistry, 2000, 8(7): 1697-1706.
);You may like
Related articles And Qustion
Lastest Price from Capecitabine manufacturers

US $0.00/KG2024-04-22
- CAS:
- 154361-50-9
- Min. Order:
- 2KG
- Purity:
- USP34
- Supply Ability:
- 20tons

US $0.00/kg2024-04-15
- CAS:
- 154361-50-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20tons