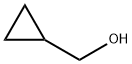
Cyclopropyl carbinol: properties, applications in organic synthesis and safety
General Description
Cyclopropyl carbinol is a slightly yellow liquid with a camphor-like odor. It is soluble in water and miscible with various organic solvents. Cyclopropyl carbinol is used as an intermediate in organic synthesis, particularly in the production of benzo-fused nitrogen rings and (hetero)aryl-fused cyclohexa-1,3-dienes. These compounds have significant applications in the development of biologically active compounds and pharmaceuticals. However, caution must be exercised when handling cyclopropyl carbinol due to its flammability and potential to cause irritation. Proper storage, protective equipment, and ventilation are necessary for safe usage.

Figure 1. Cyclopropyl carbinol
Properties
Cyclopropyl carbinol is a chemical compound with the molecular formula C4H8O. It has a density of 0.89 g/mL at 25°C, a melting point of -60°C, and a boiling point of 123-124°C at 738 mmHg. It is miscible in water and has a vapor pressure of 6 mmHg at 25°C. The refractive index of cyclopropyl carbinol is 1.431 (lit.) at 20°C. Cyclopropyl carbinol is a slightly yellow liquid. It has a camphor-like odor and is soluble in water, alcohol, acetone, ether, and benzene. Cyclopropyl carbinol is an important intermediate for the synthesis of various organic compounds. It can be prepared by the dehydration cyclization of butylene glycol in the presence of acid catalysts or by the catalytic cracking of cyclopropyl methyl ether. The former method is more commonly used because it uses a cheaper starting material and provides higher yields. Cyclopropyl carbinol is slightly more acidic than acetic acid due to the presence of a hydroxyl group, but it does not form an acid addition salt under normal conditions. 1
Applications in organic synthesis
Benzo-fused nitrogen ring synthesis
Cyclopropyl carbinol is valuable in synthesizing benzo-fused nitrogen rings, crucial structural elements found in biologically active compounds and pharmaceuticals. It serves as a precursor to cyclopropylamine derivatives, important building blocks for constructing these nitrogen-containing ring systems. Through intramolecular cyclization reactions with appropriate reagents or catalysts, these derivatives form the desired benzo-fused nitrogen rings. The cyclopropyl group in cyclopropyl carbinol plays a key role by offering unique reactivity and stereochemical control during ring formation. Its inclusion enables the introduction of a three-membered cyclopropyl ring, leading to the creation of complex chemical structures. Incorporating cyclopropyl carbinol in benzo-fused nitrogen ring synthesis provides advantages such as mild reaction conditions, high yields, and potential compound modification. This methodology contributes to exploring and developing novel bioactive molecules with potential pharmaceutical applications. 2
(Hetero)aryl-fused cyclohexa-1,3-dienes synthesis
Cyclopropyl carbinol is used in the synthesis of (hetero)aryl-fused cyclohexa-1,3-dienes through opening cyclization. This process leads to the formation of α-alkylidene-γ-butyrolactones (ABLs) with high chemoselectivity and stereoselectivity. Under the influence of Bi(OTf)3, cyclopropyl carbinyl cations are formed, which undergo ring-opening reactions and are trapped by neighboring ester groups. Subsequent hydrolysis and methanol elimination result in the formation of ABLs, mainly in the E-isomer form. The outcome of the reaction is influenced by substituents on both the carbinol and the cyclopropane. Weakly stabilizing or electron-poor donor groups on the cyclopropane lead to higher yields of ABLs, while highly stabilizing donors give competing products. A predictive model based on density functional theory calculations has been developed to determine the E/Z selectivity. Overall, this synthetic approach provides a controlled and versatile method for the production of (hetero)aryl-fused cyclohexa-1,3-dienes and valuable ABLs for various applications in organic synthesis. 3
Safety
Cyclopropyl carbinol is a volatile and flammable liquid that can cause irritation to the eyes, skin, and respiratory system. It should be handled with caution to prevent fire and explosion. In case of contact with eyes or skin, rinse immediately and seek medical help if necessary. Store cyclopropyl carbinol in a cool, dark place away from heat sources and incompatible substances. Wear appropriate protective equipment and ensure good ventilation when working with this chemical. Proper handling and storage are crucial for safety and to avoid accidents. 4
Reference
1. PubChem. Compound Summary: Cyclopropyl carbinol. National Library of Medicine, 2005, CID: 75644.
2. Kothandaraman P, Huang C, Susanti D, Rao W, Chan PW. Cyclopropyl carbinol rearrangement for benzo-fused nitrogen ring synthesis. Chemistry, 2011, 17(36):10081-10088.
3. Sandridge MJ, McLarney BD, Williams CW, France S. α-Alkylidene-γ-butyrolactone Formation via Bi(OTf)3-Catalyzed, Dehydrative, Ring-Opening Cyclizations of Cyclopropyl Carbinols: Understanding Substituent Effects and Predicting E/Z Selectivity. J Org Chem, 2017, 82(20):10883-10897.
4. Safety Data Sheets: Cyclopropyl carbinol. Fishersci, 2009, No. AC296820000.
);You may like
Related articles And Qustion
See also
Lastest Price from Cyclopropyl carbinol manufacturers
US $100.00-1.00/KG2024-03-25
- CAS:
- 2516-33-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available

US $0.00/KG2023-07-25
- CAS:
- 2516-33-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 50000KG/month