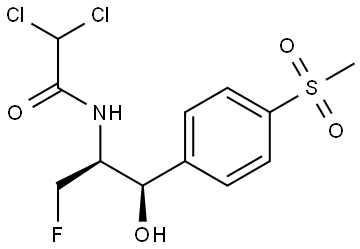
Description, Bioactivity and Application of Florfenicol
General description
Fluphenicol shows white or white-like crystalline powder, odorless, and bitter taste. It is easily dissolved in dimethylformamide and methanol, slightly dissolved in water, glacial acetic acid or chloroform. The analysis of its content eligibility criteria is as follows: its content (dry product calculation) is ≥98%, moisture 0.5% or less and residue is ≤0.1%, the heavy metal is ≤20ppm, the acidity is 4.5-6.5, the chloride is≤0.02%, the fluorine is 4.8% or higher, and the total amount of impurity spots in relevant materials is ≤2%. Florfenicol is a special antibiotic for animals. It is also used for bacterial diseases caused by sensitive bacteria in pigs, chickens and fish, especially for respiratory system infection and intestinal infection.
Pharmacological effects
Florfenicol belongs to the broad spectrum of antibacterial drugs, gram-positive bacteria and gram-negative bacteria have an inhibitory effect. In vitro, the antibacterial activity of flfenicol was significantly better than that of chloramphenicol and sulfoxycin (MIC was about 10 times), and there was no potential risk of aplastic anemia caused by flfenicol [3]. They are also a class of antibiotic drugs, which have broad spectrum bacteriostatic effect by inhibiting peptiyltransferase activity. Its antibacterial spectrum is very broad, including a variety of Gram-positive, negative bacteria and mycoplasma, etc. Susceptible bacteria include haemophilus of cattle and swine, Shigella dysentery, Salmonella, Escherichia coli, pneumococcus, influenzae, streptococcus, Staphylococcus aureus, chlamydia, leptospirosis, rickettsia, etc [4].
Application
Florfenicol has been used in the treatment of systemic infection in livestock, poultry and aquatic animals, and has a remarkable effect on respiratory system infection and intestinal infection.
Poultry: respiratory and intestinal infections caused by escherichia coli, duck serositis, mycoplasma and salmonella.
Livestock: swine, cattle, sheep and other infectious pleurisy, asthma, streptococcus disease, edema disease, piglet red dysentery.
Aquatic products: eel, debonding septicemia (unique curative effect), Edwardsiella disease, red skin disease, enteritis, etc [5].
Crab kind: attach limb cankerous disease, yellow gill, rotten gill, red leg, fluorescent phritis and red body syndrome to wait.
Turtle: red neck disease, furuncle sore, perforation disease, rot skin disease, enteritis, mumps bacterial septicemia, etc.
Frogs: cataract syndrome, ascites disease, sepsis, enteritis, etc. Fish: enteritis, ascites, vibriosis, Edwardsiellosis, etc.
Points to note when taking florfenicol
When mixing with water, if the temperature is low, a small amount of hot water can be used to stir and dissolve first, and then add water to the specified concentration.
Avoid compatibility with alkaline drugs. Combined with ampicillin, cefradine, cephalexin and so on, the curative effect is reduced. With kanamycin, streptomycin, sulfonamides, quinolones, furans, toxicity increased. When combined with VB10 and VB12, it inhibits erythropoiesis [6].
It has immunosuppressive effect and should not be used during vaccine free period.
Cows during lactation and pregnancy are prohibited.
Florfenicol interacts with drugs
Combined with ampicillin, cefradine, cephalexin and so on, the curative effect is reduced. Moreover, compatibility with kanamycin, streptomycin, sulfonamides, quinolones, and furans increases toxicity. In addition, when combined with VB10 and VB12, it inhibits erythropoiesis [7].
Pharmacological effects of florfenicol
This product is a synthetic fluoro derivative of sulfoxycin, which has demonstrated effects on a variety of gram-positive bacteria, gram-negative bacteria and mycoplasma. Pharmacokinetics showed that the drug was absorbed quickly, widely distributed, with a long half-life and high blood concentration, which could be maintained for a long time in the body [8, 9].
Derivatives of florfenicol
(1) Florfenicol sodium succinate
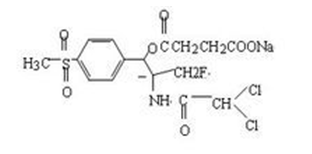
Fig. 1 The structure of florfenicol sodium succinate.
Florfenicol sodium succinate is a white or white crystalline powder, odorless and tasteless, easy to deliquescence when the air humidity is high. This product is insoluble in acetone, slightly soluble in ethanol, soluble in water. The content shall be calculated as dry goods, not less than 95%. This product is a derivative of florfenicol, designed according to the principle of prodrug, it has effects on a variety of gram-positive bacteria and gram-negative bacteria and mycoplasma. Its antibacterial mechanism is mainly through binding to the subunit of the 50S ribosome, blocking the key enzyme required for protein synthesis -- peptidyl transferase, thus specifically preventing the binding of aminoacyl tRNA to the receptor on the ribosome, inhibiting the extension of the peptide chain and preventing the synthesis of bacterial protein. The MIC50 and MIC90 of the pathogenic bacteria of bovine respiratory disease were (μg/ml): Pasteurella hemolyticus 0.50, 1.00, Pasteurella multocide 0.50, 0.50, and for actinobacillus pleuropneumoniae porcine, 0.2-1.56μg/ml [10]. This product not only increases its water solubility, but also minimizes the in vivo process loss of florfenicol after oral and injection. Esters and florfenicol are released through the metabolic breakdown of enzymes in the body, and esters overcome bacterial resistance. Fluorfenicol is targeted, and both exert pharmacological effects on the organism. Flufenicol succinate sodium showed small dosage, quick effect, long half-life, high blood concentration, can maintain blood concentration for a long time. Its main excretion is through the kidney. Flubenicol sodium succinate is used in soluble powder, oral liquid, injection and other dosage forms [11].
(2) 70% water-soluble florfenicol
Florfenicol has a lot of practical value, but there is still a difficult problem, that is, it is difficult to dissolve in water, low utilization rate, cannot give full play to its efficacy, this problem has not been very good solution. At present, the water-soluble technology of fluorphenicol in China is physical method involving micropowder of cosolvent, β -cyclodextrin inclusion, hydroxypropyl β -cyclodextrin inclusion, PVPK dispersion, PEG6000 dispersion, ultrafine, grinding and so on. However, the effect of these methods is generally not ideal, the dissolution rate is slow, the dissolution degree is small, it is difficult to meet the needs of preparation and concentration. Therefore, some scientists developed a derivative of florfenicol-70% water-soluble florfenicol [12].
This product appears as white or quasi-white powder, dissolved in water. This product is a broad - spectrum antimicrobial. Mainly compared with fbenicol, it can strongly interfere with the synthesis of bacterial proteins, with rapid absorption, wide distribution in the body, long half-life, no aplastic side effects, no resistance to drug resistance, no residue, no cross-resistance. It is a new generation of chloramphenicol antibiotics to replace chloramphenicol and methionomycin. It is mainly used for bacterial diseases and mycoplasma infection caused by sensitive bacteria in pigs, chickens and fish, and has unique curative effect on diseases caused by escherichia coli in livestock and poultry, pig infectious pleuropneumonia and panting disease. Usage and dosage are as follows: mixed feeding: livestock and poultry 1g of this product mixture 10-20kg, free to eat. First doubling, continuous feeding for 1 week. Drinking water: livestock and poultry this product 1g plus water 10-20kg, free drinking water. Continuous use for 1 week. First double, prevention halve [13].
Florfenicol is a sensitive drug
Florfenicol meets metal cations to form insoluble complexes. It is a structural homolog of chloramphenicol. Its action mechanism and antibacterial spectrum are similar to that of chloramphenicol and sulfoxycin. It can bind to the 50S subunit and inhibit the phthalyl transferase, thus preventing the extension of the phthalyl chain and interfering with protein synthesis. Florfenicol replaces -OH at the 3-carbon position of the propane chain in chloramphenicol and methanamycin with F atom in structure (see Figure 1), preventing the acetylation of bacterial acetyl transferase at this position, so it is not affected by the enzyme and is inactivated. Acetyl transferase is also related to plasmid-mediated resistance of bacteria to chloramphenicol and sulfoxycin, so florfenicol does not produce resistance similar to that mediated by chloramphenicol and sulfoxycin [14].
Compatibility with folded florfenicol
Combined with neomycin, doxycycline hydrochloride, mycelitin sulfate, lorisin, the curative effect was enhanced.
Combined with ampicillin, cefradine, cephalexin and so on, the curative effect is reduced [15].
With kanamycin, streptomycin, sulfonamides, quinolones, toxicity increased.
When combined with VB12, it inhibits erythropoiesis.
References
[1] P. Adams, K. Varma, T. Powers, J. Lamendola, Tissue concentrations and pharmacokinetics of florfenicol in male veal calves given repeated doses, American Journal of Veterinary Research 48(12) (1987) 1725-1732.
[2] N. Afifi, K.A. El‐Sooud, Tissue concentrations and pharmacokinetics of florfenicol in broiler chickens, British poultry science 38(4) (1997) 425-428.
[3] A. Anadon, M.A. Martínez, M. Martinez, A. Rios, V. Caballero, I. Ares, M.R. Martínez-Larrañaga, Plasma and tissue depletion of florfenicol and florfenicol-amine in chickens, Journal of agricultural and food chemistry 56(22) (2008) 11049-11056.
[4] Y.-q. Gao, N.-y. Gao, Y. Deng, D.-q. Yin, Y.-s. Zhang, Degradation of florfenicol in water by UV/Na2S2O8 process, Environmental science and pollution research 22(11) (2015) 8693-8701.
[5] T.E. Horsberg, K.A. Hoff, R. Nordmo, Pharmacokinetics of florfenicol and its metabolite florfenicol amine in Atlantic salmon, Journal of Aquatic Animal Health 8(4) (1996) 292-301.
[6] R. Lobell, K. Varma, J. Johnson, R. Sams, D. Gerken, S. Ashcraft, Pharmacokinetics of florfenicol following intravenous and intramuscular doses to cattle, Journal of veterinary pharmacology and therapeutics 17(4) (1994) 253-258.
[7] B.-K. Park, J.-H. Lim, M.-S. Kim, Y.-H. Hwang, H.-I. Yun, Pharmacokinetics of florfenicol and its metabolite, florfenicol amine, in dogs, Research in veterinary science 84(1) (2008) 85-89.
[8] B.K. Park, J.H. Lim, M.S. Kim, Y.H. Hwang, H.I. Yun, Pharmacokinetics of florfenicol and its major metabolite, florfenicol amine, in rabbits, Journal of Veterinary Pharmacology and Therapeutics 30(1) (2007) 32-36.
[9] B.K. Park, J.H. Lim, M.S. Kim, H.I. Yun, Pharmacokinetics of florfenicol and its metabolite, florfenicol amine, in the Korean catfish (Silurus asotus), Journal of veterinary pharmacology and therapeutics 29(1) (2006) 37-40.
[10] J.V. Samsonova, A. Cannavan, C.T. Elliott, A critical review of screening methods for the detection of chloramphenicol, thiamphenicol, and florfenicol residues in foodstuffs, Critical Reviews in Analytical Chemistry 42(1) (2012) 50-78.
[11] D.P. Schumacher, J.E. Clark, B.L. Murphy, P.A. Fischer, An efficient synthesis of florfenicol, The Journal of Organic Chemistry 55(18) (1990) 5291-5294.
[12] S. Schwarz, C. Kehrenberg, B. Doublet, A. Cloeckaert, Molecular basis of bacterial resistance to chloramphenicol and florfenicol, FEMS microbiology reviews 28(5) (2004) 519-542.
[13] J. Shen, D. Hu, X. Wu, J. Coats, Bioavailability and pharmacokinetics of florfenicol in broiler chickens, Journal of Veterinary Pharmacology and Therapeutics 26(5) (2003) 337-341.
[14] K. Varma, P. Adams, T. Powers, J. Powers, J. Lamendola, Pharmacokinetics of florfenicol in veal calves, Journal of Veterinary Pharmacology and Therapeutics 9(4) (1986) 412-425.
[15] D.G. White, C. Hudson, J.J. Maurer, S. Ayers, S. Zhao, M.D. Lee, L. Bolton, T. Foley, J. Sherwood, Characterization of chloramphenicol and florfenicol resistance in Escherichia coli associated with bovine diarrhea, Journal of Clinical Microbiology 38(12) (2000) 4593-4598.
);You may like
See also
Lastest Price from Florfenicol manufacturers
US $0.00/kg2024-04-29
- CAS:
- 73231-34-2
- Min. Order:
- 25kg
- Purity:
- ≥98%
- Supply Ability:
- 10tons
![73231-34-2 2,2-Dichloro-N-[(1R,2S)-3-fluoro-1-hydroxy-1-(4-methylsulfonylphenyl)propan-2-yl]acetamide](/ProductImageEN/2024-04/Small/ab5da5a3-d28d-4a20-88bd-f000161cfec0.png)
US $6.00/KG2024-04-25
- CAS:
- 73231-34-2
- Min. Order:
- 1KG
- Purity:
- More than 99%
- Supply Ability:
- 2000KG/Month