Diphenylmethane: properties, applications and safety
General Description
Diphenylmethane is a colorless liquid with a pleasant aromatic odor, with properties including solubility in organic solvents, stability, UV absorption and reactivity. It has applications in catalytic reactions and sonolysis, with potential for toluene disproportionation and formation of polymers similar to crosslinked polystyrene. However, it should be handled with caution due to potential irritant effects on the skin, eyes and respiratory system. Fire and explosion prevention measures are necessary during storage and usage. Good ventilation is recommended when using it, as its irritating odor threshold is low.

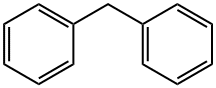
Figure 1. Diphenylmethane
Properties
Diphenylmethane is a derivative of benzene with two phenyl groups bonded to the methane carbon. It is a colorless liquid with a boiling point of 246°C. Diphenylmethane is insoluble in water but soluble in organic solvents. It has a low viscosity and is difficult to volatize at room temperature. Diphenylmethane has a high chemical stability and is difficult to react with other substances. One of the notable properties of diphenylmethane is its aromatic odor. It possesses a characteristic sweet, floral scent, which makes it a common ingredient in the formulation of perfumes and fragrances. In terms of chemical reactivity, diphenylmethane is relatively stable under normal conditions. However, it can undergo various types of reactions, including oxidation, reduction, and halogenation. It can also participate in condensation reactions to form larger, more complex molecules. Diphenylmethane exhibits moderate UV absorption properties, making it useful in sunscreen formulations as a potential UV filter or absorber. Its ability to absorb UV radiation helps protect the skin from the harmful effects of the sun. In summary, diphenylmethane is a white crystalline compound with a pleasant aromatic odor. It has a range of chemical properties, including solubility in organic solvents, stability, UV absorption, and reactivity. 1
Applications
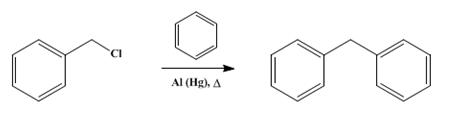
Diphenylmethane and its derivatives have been extensively studied for their applications in catalytic reactions and sonolysis. Despite not being directly detected as true reaction intermediates, recent research has provided valuable insights into the DPM-mediated reaction mechanism. It has been observed that DPM species can accumulate within zeolite pores and subsequently decompose. In toluene reactions, high concentrations of (di)methylated DPM species are formed in ZSM-5 channels, but they are unstable and decompose, resulting in the production of xylene at 200 degrees C. Furthermore, diphenylmethane has been investigated under ultrasound conditions, leading to the formation of a solid polymer resembling crosslinked polystyrene. The sonolysis process involves the dissociation of DPhM molecules inside cavitating bubbles. Secondary radical scavenging and radical recombination processes contribute to the formation of the sonopolymer in the liquid phase. In summary, diphenylmethane and its derivatives show promising applications in catalytic reactions, particularly toluene disproportionation, and sonolysis processes that result in the formation of polymers similar to crosslinked polystyrene. Further research is warranted to explore and optimize these applications effectively. 2
Safety
Diphenylmethane is a substance that should be handled with caution due to its potential irritant effects on the skin and eyes. Prolonged or repeated exposure to high concentrations of diphenylmethane may lead to skin, eye, and respiratory system irritation. In addition, there is a possibility of central nervous system stimulation and liver damage if it is absorbed into the body. Therefore, workers should avoid direct skin contact and take measures to minimize their exposure to this substance. It is crucial to prevent fires and explosions during the use and storage of diphenylmethane. The substance is combustible, so it should be stored in a cool, well-ventilated place, away from ignition sources. It is also important to work in a well-ventilated environment when using diphenylmethane in small amounts, as its irritating odor threshold is low. Overall, Diphenylmethane's safety considerations include avoiding skin contact, preventing prolonged exposure, taking precautions against fires and explosions, working in well-ventilated areas, and complying with safety guidelines provided by the manufacturer and local regulations. 3
Reference
1. PubChem. COMPOUND SUMMARY: Diphenylmethane. National Library of Medicine, 2005, CID:7580.
2. Svelle S, Olsbye U, Lillerud KP, Kolboe S, Bjørgen M. Diphenylmethane-mediated transmethylation of methylbenzenes over H-zeolites. J Am Chem Soc, 2006, 128(17):5618-5619.
3. Api AM, Belsito D, Botelho D, et al. RIFM fragrance ingredient safety assessment, diphenylmethane, CAS Registry Number 101-81-5. Food Chem Toxicol, 2023, 176 Suppl 1:113797.
);You may like
Related articles And Qustion
See also
Lastest Price from Diphenylmethane manufacturers

US $0.00-0.00/KG2024-04-28
- CAS:
- 101-81-5
- Min. Order:
- 25KG
- Purity:
- 99 %
- Supply Ability:
- 10 tons
US $50.00-1.00/KG2024-03-25
- CAS:
- 101-81-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available