Drawing of the HCN Lewis structure
Composition of the HCN Lewis structure
The HCN Lewis structure consists of three atoms: hydrogen, carbon and nitrogen. The carbon atom (C) is the central atom, which is surrounded by a hydrogen atom (H) and an oxygen atom (O). There is a triple bond between the carbon (C) and nitrogen (N) atoms and a single bond between the carbon (C) and hydrogen (H) atoms. The nitrogen (N) atom has one lone pair of electrons. It is a polar molecule with a bond angle of 180 degrees. The structural formula is shown below:
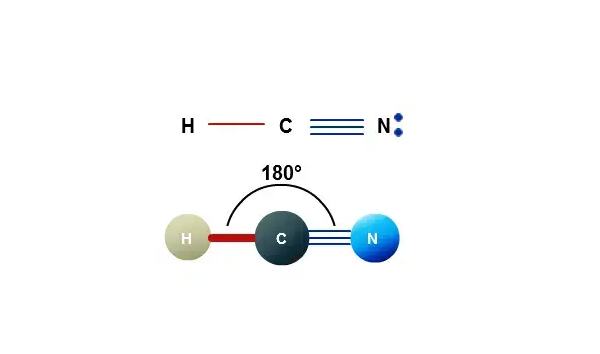
Steps for drawing the HCN Lewis structure
Step 1 Calculate the number of valence electrons of an atom
Determine the valence electrons of each atom in HCN from the periodic table, so the valence electron present in hydrogen is 1, the valence electron present in carbon is 4, and the valence electron present in nitrogen is 5. The total number of valence electrons in the HCN molecule = valence electrons of 1 hydrogen atom + valence electrons of 1 carbon atom + valence electrons of 1 nitrogen atom = 1 + 4 + 5 = 10.
Step 2 Identify the central atom
The central atom is required to have a high valence, or minimal electronegativity. So in the HCN Lewis structure the carbon atom (C) is the central atom and the nitrogen atom (N) is the external atom.
Step 3 Labelling the electron lone pairs between atoms
When we label the 10 valence electrons on each of the three atoms, carbon, hydrogen, and nitrogen, there is one electron for hydrogen, four electrons for carbon, and five electrons for nitrogen.
Step 4 Stability of structure and minimize charges on atoms by converting lone pairs to bonds
After forming a single bond with hydrogen, C has only three valence electrons left, as it shares one electron with hydrogen. Therefore, carbon will share its remaining three electrons with nitrogen to complete its octet, thus forming a triple bond between carbon and nitrogen.
);