Estradiol Valerate: Pharmacodynamic Effects, Pharmacokinetics and Dosage and Administration
General Description
Estradiol valerate is a contraceptive for women, exerting effects via estrogen receptors with limited progesterone binding. It shows progestogenic and antiandrogenic effects, with minimal impact on most haemostatic parameters compared to ethinylestradiol. Pharmacokinetics reveal absorption through the intestinal mucosa, metabolism into estradiol and valeric acid, with steady serum levels maintained over 28 days. The majority of Estradiol valerate is protein-bound, metabolized in the gastrointestinal mucosa and liver, and excreted mainly in urine. The recommended dosage of Estradiol valerate involves a 28-day regimen starting on the first day of menstrual bleeding. Contraindications include thrombosis, hepatic disease, and hypersensitivity. Consult local prescribing information for specific details on contraindications, drug interactions, and special populations.

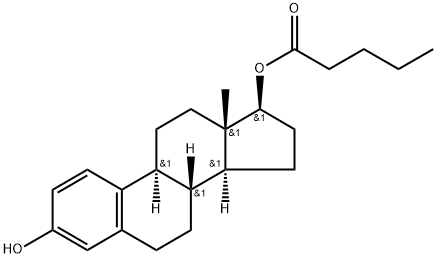
Figure 1. Estradiol valerate
Pharmacodynamic Effects
Estrogens exert their effects via estrogen receptors located throughout the female reproductive tract, bone, brain, and other tissues. Estradiol valerate/dienogest has limited binding affinity to progesterone and other receptors, demonstrating progestogenic and antiandrogenic effects. Studies on oral contraceptive formulations of estradiol valerate/dienogest and ethinylestradiol/levonorgestrel showed no clinically relevant effects on most haemostatic parameters. Fibrinogen levels varied, and D-dimer levels remained within the normal range for both formulations, but the change from baseline was greater with ethinylestradiol/levonorgestrel. Lipid parameter effects were minimal, except for HDL2-C levels, which decreased for both formulations. Triglyceride and VLDL cholesterol levels increased in both groups. SHBG levels increased significantly for both formulations, with ethinylestradiol/levonorgestrel exceeding the normal range. Cortisol-binding globulin levels also increased for both treatments but remained within the normal range. Overall, estradiol valerate/dienogest and ethinylestradiol/levonorgestrel demonstrated similar effects on haemostatic and lipid parameters, with differences observed in specific parameters such as D-dimer levels and HDL2-C. 1
Pharmacokinetics
Estradiol Valerate is a medication that is absorbed through the intestinal mucosa after oral administration. It undergoes cleavage into estradiol and valeric acid during absorption or first-pass metabolism. Only about 3% of the oral dose is directly bioavailable as estradiol. Maximum serum concentrations of estradiol are achieved in approximately 6 hours on day 1 and 3 hours on day 24, with values of 0.0706 ng/mL and 0.0660 ng/mL respectively. Estrone and estrone sulfate also reach peak concentrations within a few hours. Throughout a 28-day treatment period, steady levels of estradiol are maintained. The majority of Estradiol valerate in the serum is bound to proteins, with minimal changes observed in protein levels during the study. Following intravenous administration, the apparent volume of distribution is around 1.2 L/kg. Estradiol metabolism primarily occurs in the gastrointestinal mucosa and liver, with about 95% of the oral dose being metabolized before entering the systemic circulation. The plasma half-life of Estradiol valerate is approximately 1.5 hours, while the terminal elimination half-life ranges from 13 to 20 hours due to enterohepatic recirculation. Estradiol valerate and its metabolites are mainly excreted in urine, with minimal excretion via feces. The pharmacokinetics of Estradiol valerate in individuals with hepatic or renal impairment have not been thoroughly studied yet. 2
Dosage and Administration
Estradiol Valerate, in combination with dienogest, is indicated as a contraceptive for women. The recommended dosage and administration are as follows: Treatment should begin on the first day of menstrual bleeding. The tablets should be taken daily for 28 consecutive days, following the specified order, and at approximately the same time each day. The regimen includes estradiol valerate 3 mg for 2 days, followed by estradiol valerate/dienogest 2 mg/2 mg for 5 days, estradiol valerate/dienogest 2 mg/3 mg for 17 days, estradiol valerate 1 mg for 2 days, and placebo for 2 days. Contraceptive efficacy is not affected if there is a delay of less than 12 hours in taking a tablet. However, if the delay exceeds 12 hours, contraceptive efficacy may be reduced. Severe gastrointestinal disturbances such as diarrhea or vomiting may impair absorption of the medication. There are certain contraindications for using estradiol valerate/dienogest. These include conditions such as arterial or venous thrombosis, cerebrovascular accident, liver cancer, severe hepatic disease, migraine with focal neurological symptoms, hypersensitivity to the active substances or excipients, known or suspected sex-steroid influenced cancer, severe arterial or venous thrombosis risk factors, and undiagnosed vaginal bleeding. It is important to note that there is currently no data available on the efficacy and tolerability of estradiol valerate/dienogest in adolescents under 18 years of age. Detailed information regarding contraindications, drug interactions, precautions, and use in special patient populations should be consulted in the local prescribing information. 1
Reference
1. Hoy SM, Scott LJ. Estradiol valerate/dienogest: in oral contraception. Drugs. 2009; 69(12): 1635-1646.
2. Zeun S, Lu M, Uddin A, et al Pharmacokinetics of an oral contraceptive containing oestradiol valerate and dienogest. Eur J Contracept Reprod Health Care 2009 Jun; 14(3): 221-232.
);You may like
Related articles And Qustion
Lastest Price from Estradiol valerate manufacturers

US $9.00-60.00/g2024-04-26
- CAS:
- 979-32-8
- Min. Order:
- 10g
- Purity:
- 99%
- Supply Ability:
- 10 tons

US $20.00-10.00/kg2024-04-26
- CAS:
- 979-32-8
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 20