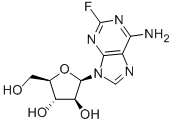
Fludarabine, cyclophosphamide and rituximab (FCR)
It is estimated that over 15,000 new patients will be diagnosed with chronic lymphocytic leukemia (CLL), and approximately 5000 will die from this disease in the United States in 2016. CLL is a disease more commonly diagnosed in the elderly (median age 72 years), male (2:1 male to female ratio), and white populations. Combining fludarabine, cyclophosphamide, and rituximab (FCR) is a widely used CIT regimen for CLL[1].
Fludarabine and cyclophosphamide are chemotherapy drugs. Rituximab is a type of targeted cancer drug called a monoclonal antibody.
Fludarabine, a fluorinated purine nucleoside analog that is resistant to deamination by adenosine deaminase, was initially studied as a single agent in patients with relapsed/refractory (R/R) CLL, yielding responses of 33–57%. In previously untreated patients, overall response rates (ORRs) of single-agent fludarabine were as high as 70%[2].
In vitro studies indicate that fludarabine may enhance the effect of cyclophosphamide by inhibiting the repair of the interstrand cross-links produced by the alkylating agent. In an attempt to improve response rates with fludarabine alone and based on in vitro evidence of synergistic activity between fludarabine and cyclophosphamide, a regimen combining these two agents (FC) was tested as a therapy for CLL, including patients who were previously exposed to fludarabine as a single agent. A total of 128 patients with CLL were treated with different FC schedules. Patients not refractory to fludarabine or alkylating agents at entry into the study had an overall response rate of ⩾80%. The synergy between these two agents was further evidenced by patients who were refractory to fludarabine when entering the study and still achieved a 38% response rate following the FC combination. However, complete response (CR) rates remained low, at 35% for previously untreated patients.
Rituximab, a chimeric, monoclonal antibody targeting CD20, increased the cytotoxicity of fludarabine and cyclophosphamide in lymphoma cell lines. This provided the rationale for combining rituximab with the FC regimen (FCR). The FCR regimen was initially evaluated in a phase II trial by the MD Anderson group in patients with R/R CLL.
References
[1] Róbert Szász, Árpád Illés, Béla Telek. “Fludarabine-Cyclophosphamide-Rituximab Treatment in Chronic Lymphocytic Leukemia, Focusing on Long Term Cytopenias Before and After the Era of Targeted Therapies.” Pathology Oncology Research (2021): 1609742.
[2] Alan P Skarbnik, Stefan Faderl. “The role of combined fludarabine, cyclophosphamide and rituximab chemoimmunotherapy in chronic lymphocytic leukemia: current evidence and controversies.” Therapeutic Advances in Hematology 8 3 (2017): 99–105.
);You may like
Related articles And Qustion
See also
Lastest Price from Fludarabine manufacturers

US $50.00/kg2024-04-23
- CAS:
- 21679-14-1
- Min. Order:
- 1kg
- Purity:
- 99.10%
- Supply Ability:
- 50000kg

US $0.00/kg2024-04-15
- CAS:
- 21679-14-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20tons