Fludioxonil: pharmacokinetics, toxicity and emergency treatment
General Description
Fludioxonil, an agricultural fungicide, has specific pharmacokinetic characteristics. In goats, it was detected in various tissues and milk after oral administration. Laying hens eliminated most of the residue through excreta, but residues were found in liver, muscle, skin with fat, egg yolk, and egg white. In dairy cows, fludioxonil and its metabolites were quantifiable at high feeding levels. Dermal absorption rates were low in rats and human skin. Fludioxonil exhibits endocrine disruptor activity, stimulating miR-21 expression associated with breast cancer. It inhibits cell viability, proliferation, and motility, induces oxidative stress, and shows toxic effects when combined with other fungicides. Emergency treatment involves decontamination, artificial respiration, CPR, clear airway maintenance, oxygen provision, and management of pulmonary edema, shock, seizures, among other interventions.

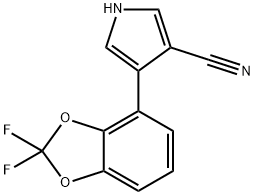
Figure 1. Fludioxonil
Pharmacokinetics
Fludioxonil, a fungicide used in agriculture, exhibits specific pharmacokinetic characteristics. In goats, radiolabeled fludioxonil was administered orally for four consecutive days. On the fourth day, radioactive residues were detected in various tissues and milk. The levels of calculated fludioxonil residue were 0.07 mg/kg in tenderloin muscle, 0.19 mg/kg in fat, 5.8 mg/kg in liver, 2.9 mg/kg in kidney, and 2.2 mg/kg in milk. In laying hens, gelatin capsules containing radiolabeled fludioxonil were given over eight consecutive days. The majority of the radiolabeled residue was eliminated through excreta. Residues were found in liver, muscle, skin with fat, peritoneal fat, egg yolk, and egg white. Organic solvent extractions released varying proportions of the total radiolabeled residue in different tissues and eggs. In a feeding study with dairy cows, fludioxonil and its metabolites were quantifiable only at the highest feeding level (5.5 ppm). Residues were detected in liver and kidney at concentrations of 0.014-0.017 mg/kg and 0.022-0.025 mg/kg, respectively, at this feeding level. Regarding dermal absorption, fludioxonil exhibited low absorption rates in rats in vivo (< 5%) and human skin in vitro (< 0.5%). At higher application levels, the in vitro study overestimated the absorption compared to in vivo results. 1
Toxicity
Fludioxonil exhibits endocrine disruptor activity as antiandrogens and can stimulate the expression of miR-21, an oncomiR associated with breast cancer, in human breast cancer cells. It reduces the expression of miR-21 target proteins and demonstrates specificity in regulating miRNA levels. The induction of miR-21 by fludioxonil is inhibited by various antagonists and inhibitors targeting estrogen and androgen receptors, protein synthesis, and signaling pathways. Fludioxonil inhibits cell viability, estradiol-induced proliferation, and cell motility in MCF-7 breast cancer cells. When used in combination with other fungicides such as pyrimethanil and cyprodinil, fludioxonil shows toxic effects on cellular ATP levels and mitochondrial membrane potential. It induces oxidative stress by reducing cellular thiols and increasing the expression of reactive species formation-related enzymes. The neuronal SH-SY5Y cell line is more vulnerable to toxicity, exhibiting increased caspase-3 expression indicative of apoptosis initiation. Cyprodinil is the most toxic agent individually, and there may be some synergy when these agents are combined. These findings suggest that fludioxonil has endocrine-disrupting properties, can influence miRNA expression associated with breast cancer, and exhibits toxic effects on cellular energy metabolism, mitochondrial function, and oxidative stress responses in neuronal and glial cells. 2
Emergency treatment
Emergency treatment for fludioxonil exposure involves immediate and subsequent interventions. Immediate first aid includes decontamination, artificial respiration if necessary, and CPR if the patient is not breathing. Contaminated eyes should be flushed with water, while vomiting should be avoided. Seek medical attention promptly. Basic treatment focuses on ensuring a clear airway, monitoring respiratory function, providing oxygen, and managing pulmonary edema, shock, and seizures. Eye contamination should be addressed using saline irrigation during transport. Emesis should not be induced, and mouth rinsing may be done with water. Skin burns should be covered after decontamination. Advanced treatment may include intubation for airway control, positive-pressure ventilation, drug therapy for pulmonary edema and bronchospasm, cardiac arrhythmia monitoring and treatment, intravenous fluid administration, seizure management, and eye irrigation assistance. 3
Reference
1. WHO/FAO. Joint Meeting on Pesticide Residues; Pesticide Residues in Food: Fludioxonil (131341-86-1). 2004, 74-96.
2. Teng Y, Manavalan TT, Hu C, Medjakovic S, Jungbauer A, Klinge CM. Endocrine disruptors fludioxonil and fenhexamid stimulate miR-21 expression in breast cancer cells. Toxicol Sci, 2013, 131(1):71-83.
3. Currance PL, Clements B, Bronstein AC. Emergency Care For Hazardous Materials Exposure. 3rd revised edition, Elsevier Mosby, St. Louis, 2007, 160-161.
);You may like
Related articles And Qustion
Lastest Price from Fludioxonil manufacturers
US $10.00-2.00/KG2024-04-28
- CAS:
- 131341-86-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available

US $9.90/kg2024-02-27
- CAS:
- 131341-86-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1000kg