FMoc-Val-Cit-PAB: Applications of FMoc-Val-Cit-PAB in Antibody-Drug Conjugates and Its Synthesis Method
General Description
FMoc-Val-Cit-PAB is a versatile peptide conjugate used in drug delivery, featuring a Val-Cit linker for controlled drug release and a PAB moiety for enhanced stability. In antibody-drug conjugates (ADCs), it serves as a sulfatase-cleavable linker, improving cytotoxicity against target cells while maintaining selectivity. The synthesis method involves preparing FMoc-Cit-PABOH, adding piperidine, and then FMoc-Val-OSu to create the desired compound. This synthesis route offers a higher yield of 85% and avoids epimerization, making it a reliable option for ADC research. Overall, FMoc-Val-Cit-PAB shows great promise in targeted drug delivery systems, particularly in cancer therapy.

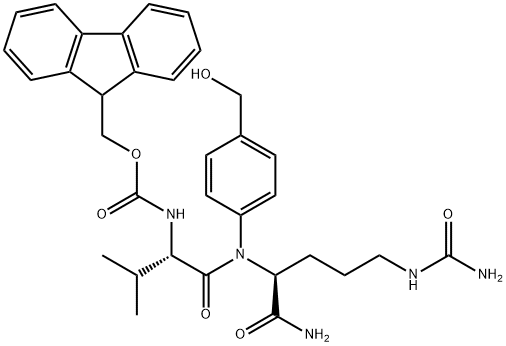
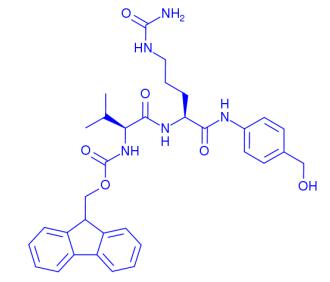
Figure 1. FMoc-Val-Cit-PAB
Overview
FMoc-Val-Cit-PAB is a peptide conjugate that combines the amino acids valine (Val) and citrulline (Cit) with a para-aminobenzoic acid (PAB) moiety. This peptide conjugate finds its application in the field of drug delivery and conjugation chemistry. The FMoc group serves as a protecting group that allows for facile synthesis and purification. The Val-Cit sequence provides a protease-cleavable linker, enabling controlled release of conjugated drugs at target sites. The PAB moiety serves as a chelating agent for metal ions, enhancing the solubility and stability of the conjugate. Overall, FMoc-Val-Cit-PAB offers a versatile platform for the development of targeted drug delivery systems. 1
Applications in antibody-drug conjugates
FMoc-Val-Cit-PAB, a sulfatase-cleavable linker, demonstrates promising applications in antibody-drug conjugates, a vital category of targeted drug delivery systems. ADCs amalgamate the cell-specificity of monoclonal antibodies (mAbs) with the cytotoxicity of small molecules, connected via covalent linkers crucial for ADC efficacy and safety. Enzyme-cleavable dipeptidic linkers have shown effectiveness in selectively releasing payloads in target cell lysosomes, yet they suffer from drawbacks like instability in rodent plasma and high hydrophobicity. In contrast, arylsulfate-containing ADC linkers, such as FMoc-Val-Cit-PAB, offer a compelling alternative. These linkers are cleaved by lysosomal sulfatase enzymes, enabling traceless payload release while bypassing instability issues associated with dipeptide linkers. Incorporating FMoc-Val-Cit-PAB into ADCs, such as ADC 2 and ADC 3 with trastuzumab and monomethyl auristatin E (MMAE) payload, led to enhanced cytotoxicity against HER2-positive cells compared to non-cleavable ADC 4, while maintaining selectivity over HER2-negative cells. The stability, solubility, and synthetic accessibility of arylsulfate linkers make them a compelling option for cleavable ADC linkers, offering advantages over conventional dipeptidic linkers. Overall, FMoc-Val-Cit-PAB demonstrates immense potential in improving the efficacy and safety profile of ADCs, highlighting its significance in targeted cancer therapy and drug delivery. 2
Synthesis method
The synthesis method for FMoc-Val-Cit-PAB, a crucial component in the development of antibody-drug conjugates, is outlined as follows: Firstly, a solution of FMoc-Cit-PABOH is prepared in dimethylformamide (DMF) at a concentration of 0.2 M. Piperidine is then added in excess (5.0 equivalents) to the solution and stirred at room temperature for 5 hours. Following the reaction, excess DMF and piperidine are removed under reduced pressure, and any remaining piperidine is eliminated through repeated co-evaporation with DMF. The resulting residue is dissolved in DMF at a concentration of 0.1 M. Next, FMoc-Val-OSu is added to the solution, and the reaction mixture is stirred at room temperature for 20 hours. After completion of the reaction, DMF is removed under reduced pressure, and the residue is purified by flash column chromatography using a solvent system of 3-12% MeOH-CH2Cl2. This methodology offers an improved route for the synthesis of the cathepsin B cleavable dipeptide linker, FMoc-Val-Cit-PAB, which is widely used in ADC research. The linker plays a crucial role in connecting the antibody and payload in ADCs, allowing for selective delivery of potent anticancer agents to tumor cells or associated microenvironments. This alternative synthesis route involves six steps from L-Citrulline and achieves a higher overall yield of 85%. Additionally, the modified route avoids undesirable epimerization, resulting in a more efficient and reliable synthesis process. 3
Reference
1. Fmoc-Val-Cit-PAB. National Center for Biotechnology Information. 2024; PubChem Compound Summary for CID 59165375.
2. Bargh JD, Walsh SJ, Isidro-Llobet A, Omarjee S, Carroll JS, Spring DR. Sulfatase-cleavable linkers for antibody-drug conjugates. Chem Sci. 2020; 11(9): 2375-2380.
3. Mondal D, Ford J, Pinney KG. Improved Methodology for the Synthesis of a Cathepsin B Cleavable Dipeptide Linker, Widely Used in Antibody-Drug Conjugate Research. Tetrahedron Lett. 2018; 59(40): 3594-3599.
);You may like
Related articles And Qustion
Lastest Price from FMoc-Val-Cit-PAB manufacturers
US $0.00/g2024-03-29
- CAS:
- 159858-22-7
- Min. Order:
- 10g
- Purity:
- >95.00%
- Supply Ability:
- 10g

US $0.00-0.00/KG2022-10-14
- CAS:
- 159858-22-7
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 1Ton