Fulvestrant: a major breakthrough in breast cancer treatment
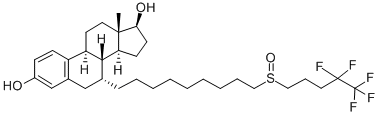
Fulvestrant, an anti-estrogenic breast cancer treatment, was approved by the US FDA in September 2002 and approved by the European Union in April 2004 for marketing in Europe. The chemical name of Fulvestrant is Estra-1,3,5(10)-triene-3,17-diol, 7-[9-[(4,4,5,5,5-pentafluoropentyl)sulfinyl]nonyl]-, (7α,17β)- (9CI, ACI), which has estrogen antagonistic activity, and has an affinity for estrogen receptors similar to that of estradiol, and is able to block the growth-stimulating effect of estradiol on human breast cancer cells. It can block the growth stimulating effect of estradiol on human breast cancer cells. Compared with tamoxifen, a widely used drug in clinical practice, the affinity of fulvestrant for estrogen receptor is 100 times higher than that of tamoxifen, and the duration of tumour growth inhibition of fulvestrant is twice as long as that of tamoxifen, and it can be more effective in the treatment of tamoxifen-resistant breast cancers[1] . The side effects of Fulvestrant are far less than those of similar drugs, and it does not cause the occurrence of endometrial cancer due to the estrogen-like effect of uterine tissues; clinical studies have found that the drug does not pass the blood-brain barrier, and does not cause side effects such as vasoconstriction, and the increase in the replacement marker of bone turnover rate has not been found; it has been proved to have a good tolerance in the phase Ⅰ and phase Ⅱ clinical studies; therefore, Fulvestrant, which has a good therapeutic effect and fewer side effects, is a good choice for the treatment of breast cancer. Therefore, the emergence of fulvestrant with good efficacy and less side effects is a major breakthrough in breast cancer treatment[2].

Figure 1.Fulvestrant
Pharmacological effects
The incidence of breast cancer is increasing at an alarming rate of 5% per year, and there is a trend of younger incidence. The goals of treatment for advanced breast cancer are to relieve symptoms, prolong survival and improve quality of life. Estrogen resistance therapy is currently the treatment of choice for estrogen receptor (ER)-positive breast cancer. Fulvestrant is a new type of estrogen receptor antagonist - estrogen receptor down-regulator class of anti-breast cancer therapeutic drugs, through binding, blocking and degrading estrogen receptors, comprehensively blocking the signaling pathway, in order to achieve the purpose of inhibition of tumour growth, and was marketed in the United States in 2002 and in China in 2011, for endocrine It was marketed in the US and China in 2002 and 2011, respectively, for postmenopausal ER-positive metastatic breast cancer (MBC) that has progressed after endocrine therapy. Currently, fulvestrant is used as the first line of endocrine therapy for advanced Luminal breast cancer. Both 250mg and 500mg of fulvestrant alone have shown good results in both second and first line endocrine therapy for postmenopausal ER-positive MBC. By binding, blocking and degrading the oestrogen receptor, the signalling pathway is comprehensively blocked in order to inhibit tumour growth[3].
Since the drugs available in breast cancer treatment have different mechanisms of action, drug combination is a reasonable way to improve the therapeutic effect. At present, there are several forms of drug combination of Fulvestrant:
1、Fulvestrant combined with other endocrine therapy
Preclinical pharmacological experiments show that fulvestrant may have higher efficacy in a low estrogen environment, thus, the combination of fulvestrant and aromatase inhibitors AIs may be the best therapy, which can produce complete estrogen blockade by down-regulating estrogen receptors and inhibiting estrogen synthesis[4].
2、Fluvestrant combined with targeted drugs
A: Combination of Fulvestrant and CDK 4/6 inhibitors
HR+ breast cancer growth is dependent on CDK4 and CDK6, which promote progression from the G1 phase to the S phase of the cell cycle.CDK4/6 inhibitors block the growth of HR+ breast cancer cells, even those resistant to endocrine therapy.Inhibition of CDK4/6 has been established as a novel therapeutic strategy to improve the efficacy of endocrine therapy and reverse primary and secondary drug resistance[4].
B: Combination of Fulvestrant with mTOR inhibitors
Inhibition of mammalian target of rapamycin (mTOR), one of the key pathways of endocrine resistance, as well as complete blockade of ER and selective estrogen receptor downregulation are emerging strategies for the treatment of endocrine resistance[4].
C: Combination of Fulvestrant and PI3K Inhibitors
Activation of the phosphatidylinositol 3-kinase (PI3K) pathway is one of the hallmarks of endocrine therapy resistance in HR+ breast cancer cells; therefore, dual blockade of the ER pathway and PI3K is a promising strategy to overcome drug resistance[4].
D: Combination of Fulvestrant and Vascular Endothelial Growth Factor Antibody
The phase III LEA trial [29] tested whether combining anti-vascular endothelial growth factor (VEGF) antibodies with endocrine therapy might delay the development of resistance[4].
E: Combining fulvestrant with EGFR-HER2 targeted therapy
Resistance to endocrine drugs may be associated with acquired overexpression of epidermal growth factor receptor (EGFR) or human epidermal growth factor receptor 2 (HER2); pre-trial data suggests that dual EGFR- and HER2-targeting drugs can re-sensitise breast cancer to endocrine therapy[4].
Mechanism of action
Fulvestrant exerts its antitumour activity by competing with endogenous oestrogen for binding to the oestrogen receptor. The relative binding affinity of fulvestrant for the oestrogen receptor is 89 per cent that of oestradiol, compared with 2.5 per cent for tamoxifen. Binding of fulvestrant to the estrogen receptor appears to prevent receptor dimerisation; as a result, binding of the receptor complex to estrogen-responsive elements is prevented and transcription is not activated. Studies have shown that the receptor complexes formed with fulvestrant are labile and sensitive to degradation. In human breast cancer cells and mouse uterine studies, this ectopic may be responsible for the reduced levels of estrogen receptors with no change in mRNA levels. In addition, it has been suggested that fulvestrant may reduce the shuttling of the estrogen receptor from the cytoplasm to the nucleus, thereby promoting its degradation[5].
Absorption, Distribution, Metabolism, and Excretion
Pharmacokinetic data suggest that intravenous fulvestrant is the most effective mode of administration, with a single dose250 of a long-acting formulation of fulvestrant administered intravenously over the dosing interval (28 ± 3 days) maintaining plasma concentrations in the range of 2-3 times higher than those required for predicted pharmacological activity. Fulvestrant is highly metabolisable and is excreted predominantly in the faeces, and pharmacokinetic studies also suggest that fulvestrant is unlikely to be the subject or cause of significant CYP3A4-mediated drug interactions[6].
Synthesis
1、Synthesis of intermediate 2
The first step in the synthesis of fulvestrant is the acetylation of nandrolone (1) at the 7-position hydroxyl group and the 2-position enolic hydroxyl group for protection. Isopropenyl acetate can also be used as an acetylation reagent for nandrolone, and the yield can reach 77%~85% under the catalysis of p-toluenesulfonic acid. This method has the advantages of simple operation, easy crystallisation and mild conditions[7].
2、Synthesis of intermediate 4
There is only one method reported in the literature for the synthesis of dienone compound 4 , i.e., the 6-position bromination of acetylnandrolone with NBS, and then directly under the condition of strong base (LiBr/Li2CO3) to remove a molecule of HBr, which then generates the dienone, with the yields ranging from 65% to 79%[8].
3、Synthesis of intermediate 6
The structure of intermediate 6 consists of a steroid nucleus, a nine-carbon aliphatic chain and a five-carbon aliphatic chain. There are two main synthesis methods, one is to connect the nine-carbon chain to the steroid and then to the five-carbon chain ; the other is to connect the nine-carbon chain to the five-carbon chain and then to react with the steroid nucleus[9].

Figure 2.Fulvestrant synthesis
Method 1: Stevenson and Macdonald in patents WO0232922 and WO015081, respectively, such as the synthesis of figure 2, from the nine-carbon chain octanediol through the sodium thiol to the five-carbon chain of pentafluoropentanol and then reacted with the steroid nucleus. The main advantages are as follows: (1) starting from the expensive dienone intermediate 4,intermediate 6 can be obtained in one step; (2) the whole reaction is carried out in solution, and the final product can be crystallised directly from the solution with a high yield; (3) the ratio of the 7-position α/β isomers is up to 2.5:1[10].
Method 2: Pettersson, in his patent WO2005077968, adopted the method of connecting the steroid to the nine-carbon chain first, and then to the five-carbon chain, which can also be used to synthesise the 6-carbon chain with high efficiency.
4、Synthesis of intermediate 7
The arylation of compound 6 can be carried out by a number of conventional methods, one of the more suitable being the reaction with copper bromide, to which a small amount of lithium bromide can be added to increase the solubility of the copper salt in solution.
5、Synthesis of intermediate 8
Acetyl group removal can also be achieved by a number of conventional methods, the most suitable of which is hydrolysis by methanol solutions of KOH or NaOH.
6、Synthesis of compound 9(fulvestrant)
Oxidation of the sulphide to the sulfoxide can be carried out with a variety of oxidising reagents such as hydrogen peroxide, peracids (e.g. periodate, 3-chloroperbenzoic acid, or peroxyacetic acid), etc. The oxidation is usually carried out under mild conditions. Oxidation is usually carried out under mild conditions to reduce the risk of over-oxidation, and a 2:1 ratio of oxidising reagent to compound 6 is generally used.
Reference
[1]Huober J,Thürlimann B.The role of combination chemotherapy in the treatment of patients with metastatic breast cancer[J].Breast Care,2009,4(6);367-372.
[2]Nagini S.Breast Cancer:current molecular therapeutic targets and new players[J].Anticancer Agents Med Chem.2017.17 (2):152-163.
[3]Jonat W,Kaufmann M,Sauerbrei W,et al.Goserelin versus cyclophosphamide,methotrexate,and fluorouracil as adju- vant therapy in premenopausal patients with node -positive breast cancer:The Zoladex Early Breast Cancer Research Association Study[J].J Clin Oncol,2002,20(24):4628 - 4635.
[4]Ponzone R,Mininanni P,Cassina E,et al.Aromatase inhibitors for breast cancer:different structures,same effects[J]. Endocr Relat Cancer,2008,15(1):27-36.
[5]Fisher B,Costantino JP,Wickerham DL,et al.Tamoxifen for prevention of breast cancer:report of the National Surgical Adjuvant Breast and Bowel Project P1 Study[J].J Nat Cancer Inst,1998,90(18):1371-1388.
[6]Bhattacharya P,Abderrahman B,Jordan VC.Opportunities and challenges of long term anti-estrogenic adjuvant thera- py:treatment forever or intermittently[J].Expert Rev Anticancer Ther,2017,17(4):297-310.
[7]Howell A,Robertson JFR,Quaresma Albano J,et al.Fulvestrant,formerly ICI 182,780,is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment[J].J Clin Oncol, 2002,20(16):3396-3403.
[8]Osborne ,Pippen J,Jones SE,et al.Double- blind,randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy:results of a North American trial[J].J Clin Oncol,2002,20(16):3386-3395
[9]Howell A,Pippen J,Elledge RM,et al.Fulvestrant versus anastrozole for the treatment of advanced breast carcino- ma:a prospectively planned combined survival analysis of two multicenter trials[J].Cancer,2005,104(2):236-239.
[10]Mehta RS,Barlow WE,Albain KS,et al.Combination anastrozole and fulvestrant in metastatic breast cancer[J].N Engl J Med,2012,367(5):435-444
);You may like
Related articles And Qustion
See also
Lastest Price from Fulvestrant manufacturers

US $30.00/kg2024-04-30
- CAS:
- 129453-61-8
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 2000kg

US $0.00/KG2024-03-16
- CAS:
- 129453-61-8
- Min. Order:
- 100g
- Purity:
- 98%+
- Supply Ability:
- 100kg