Hexamethylphosphoramide: metabolism and applications
General Description
Hexamethylphosphoramide is a chemical compound with various applications. It can be used as a promoter in the chemical synthesis of molecules, such as 3-arylated indenes, which enables efficient and facile direct arylation reactions. In addition, hexamethylphosphoramide serves as an electrolyte in Li-O2 batteries, which greatly improves their capacity, rate capability, and cycle life. Furthermore, hexamethylphosphoramide can be used as a co-solvent in NMR spectroscopy to enhance signal intensities and simplify sample preparation. However, it is important to note that inhalation exposure to hexamethylphosphoramide has been shown to induce nasal tumors in rats, emphasizing its potential toxicological implications. Overall, hexamethylphosphoramide exhibits diverse properties and multifaceted potential applications, but also warrants careful consideration when handling or exposed to it.

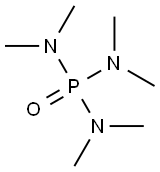
Figure 1. Hexamethylphosphoramide
![Article illustration]()
![Article illustration]() Metabolism
Metabolism
Hexamethylphosphoramide has been shown to have a considerable antispermatogenic effect on rats and mice. Despite this, little is known about its detoxification in animals. A recent study on hexamethylphosphoramide metabolism in houseflies revealed some insights into the process, but its fate in rats and mice remained unclear. Unlike tri-ethylene-phosphoramide, which is rapidly metabolized into inorganic phosphate in mice, 32P-hexamethylphosphoramide was not degraded to phosphate in either species during a preliminary investigation. Instead, most of the compound administered was excreted rapidly without being altered in any significant way. Further studies have investigated the metabolism of other hexa-alkylphosphoramides such as hexa-ethylphosphoramide, hexa-n-propylphosphoramide, and hexamethylthiophosphoramide, with some results published elsewhere. 1
![Article illustration]() Applications
Applications
Chemical synthesis
Hexamethylphosphoramide can serve as a promoter in the chemical synthesis of 3-arylated indenes. It facilitates a facile and efficient direct arylation of indenes with unactivated aryl fluorides by promoting the reaction in the presence of lithium diisopropylamide (LDA) at room temperature (rt). The use of hexamethylphosphoramide eliminates the need for very low reaction temperatures, which are traditionally required for the defluorination of aryl fluorides via aryne. The method allows for the cleavage and activation of C-F bonds in unactivated electron-rich aryl fluorides, providing an alternative approach for the synthesis of structurally diverse molecules from organofluorine compounds. Overall, hexamethylphosphoramide plays a critical role in promoting and facilitating the direct arylation reaction, thereby enabling the efficient synthesis of 3-arylated indenes. 2
Electrolyte in Li-O2 batteries
The use of hexamethylphosphoramide as an electrolyte in Li-O2 batteries has been shown to be effective in addressing some of the limitations observed in current Li-O2 batteries. Specifically, hexamethylphosphoramide can dissolve discharged Li2O2, Li2CO3, and LiOH up to certain concentrations, allowing for improved capacity, rate capability, voltaic efficiency, and cycle life in comparison to ether-based Li-O2 batteries. Moreover, the use of a LiPON-protected lithium anode in conjunction with hexamethylphosphoramide electrolyte allows for reversible cycling of the battery. Of particular importance, advanced research techniques provide evidence that the operation of the hexamethylphosphoramide-based Li-O2 battery is dominated by the nearly reversible formation/decomposition of Li2O2, with minimal side reactions occurring during battery operation. 3
Co-solvent
Hexamethylphosphoramide serves as a co-solvent in the new NMR solvent system composed of DMSO-d6 and hexamethylphosphoramide-d18. Hexamethylphosphoramide enhances the swelling and mobility of biomass samples, which results in enhanced signals of NMR spectra and improved structural analysis of biomass. The use of hexamethylphosphoramide as a co-solvent eliminates the need for derivatization or isolation of biomass, which simplifies the sample preparation process. Additionally, it enables the analysis of biomass with a room-temperature NMR probe, rather than requiring the use of cryo-probes to enhance signal intensities. 4
Induction of nasal tumors in rats
Hexamethylphosphoramide is a carcinogenic chemical that induces nasal tumors in rats through inhalation exposure. The concentration and duration of exposure to hexamethylphosphoramide determine the incidence and severity of the tumors, with concentrations of 50 to 4000 parts per billion (ppb) inducing tumors after 6 to 24 months of exposure. However, exposure at 10 ppb for 24 months did not lead to nasal tumors. The tumors were primarily epidermoid carcinomas and developed from the respiratory epithelium or subepithelial nasal gland. Squamous metaplasia or dysplasia in the anterior nasal cavity appears to play a role in the development of these tumors. Interestingly, glandular cells play an important role in the development of epidermoid carcinomas, and the neoplastic squamous cells also exhibit abundant features of glandular differentiation. The morphological expression of glandular cell metamorphosis in the tumor cells involves intermediate cells showing both glandular and squamous differentiation, inter- or intracellular lumina, secretory vesicles, and mucus droplets in squamous cells and keratin plates. Overall, hexamethylphosphoramide is a potent carcinogenic chemical that induces nasal tumors with a glandular element, which highlights the complex histological and ultrastructural features of the tumors. 5
Reference
1. Jones AR, Jackson H. The metabolism of hexamethylphosphoramide and related compounds. Biochem Pharmacol, 1968, 17(11):2247-2252.
2. Ji X, Li J, Wu M, Cao S. Facile Synthesis of 3-Arylindenes by HMPA-Promoted Direct Arylation of Indenes with Aryl Fluorides. ACS Omega, 2018, 3(8):10099-10106.
3. Zhou B, Guo L, Zhang Y, et al. A High-Performance Li-O2 Battery with a Strongly Solvating Hexamethylphosphoramide Electrolyte and a LiPON-Protected Lithium Anode. Adv Mater, 2017, 29(30):1701568.
4. Yoo CG, Pu Y, Li M, Ragauskas AJ. Elucidating Structural Characteristics of Biomass using Solution-State 2?D NMR with a Mixture of Deuterated Dimethylsulfoxide and Hexamethylphosphoramide. ChemSusChem, 2016, 9(10):1090-1095.
5. Lee KP, Trochimowicz HJ. Morphogenesis of nasal tumors in rats exposed to hexamethylphosphoramide by inhalation. Environ Res, 1984, 33(1):106-118.
);You may like
Related articles And Qustion
Lastest Price from Hexamethylphosphoramide manufacturers

US $100.00/kg2024-02-27
- CAS:
- 680-31-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100 tons

US $0.00/KG2023-11-15
- CAS:
- 680-31-9
- Min. Order:
- 25KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month