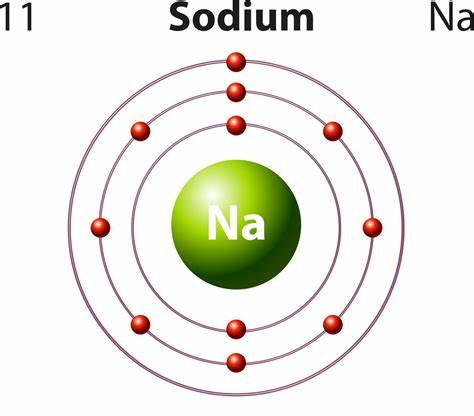
How many neutrons, protons and electrons does a sodium atom have?
What is Neutron?
A neutron is a neutral subatomic particle that, in conjunction with protons, makes up the nucleus of every atom except ordinary hydrogen (whose nucleus has one proton and no neutrons). Along with protons and electrons, it is one of the three fundamental particles making up atoms, the basic building blocks of all matter and chemistry.
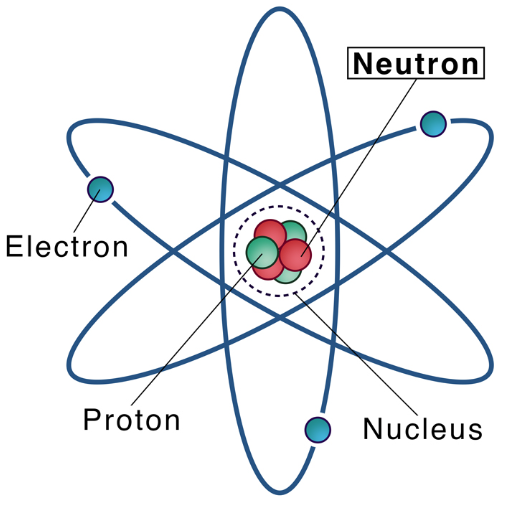
The neutron has no electric charge and a rest mass equal to 1.67492749804 × 10−27 kg—marginally greater than the proton but 1,838.68 times greater than the electron's. Neutrons and protons, commonly called nucleons, are bound together in the dense inner core of an atom, the nucleus, where they account for 99.9 percent of the atom’s mass. Developments in high-energy particle physics in the 20th century revealed that neither the neutron nor the proton is an actual elementary particle. Rather, they are composites of extremely small elementary particles called quarks. The neutron comprises two down quarks, each with 1/3 elementary charge, and one up quark, with 2/3 elementary charge.
What is the number of neutrons in sodium?
The mass number defines the number of protons and neutrons in an atom. The atomic number shows the protons in an atom (nucleus). Because there is the same number of protons as electrons in an atom (uncharged atom), this number also indicates the number of electrons in an atom. For sodium, that means there are 11 protons and 11 electrons. For any element:
number of neutrons = mass number - atomic number
For sodium,
number of neutrons = mass number - atomic number
=23-11=12
Hence, sodium contains 12 neutrons, 11 protons, and 11 electrons.
);