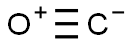How to draw the Lewis structure of CO
The Lewisian structure of CO
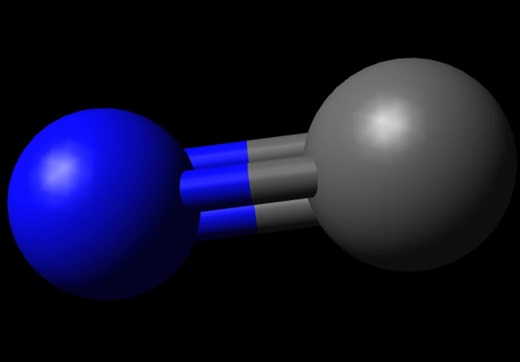
The Lewis structure of CO is made up of a carbon atom (C) and an oxygen atom (O). The carbon and oxygen atoms are connected by a triple bond with a lone electron pair on each atom. Structurally the carbon and oxygen atoms are at the same level. This is shown in the diagram below:

Steps for drawing the CO Lewis structure
Step 1 Calculate the number of valence electrons for C and O
According to the periodic table to calculate the total valence electrons in the CO molecule, carbon and oxygen are the 14th and 16th group elements on the periodic table, so the valence electron of carbon is 4, and the valence electron of oxygen is 6. The total valence electrons in CO = the valence electrons given by one carbon atom + the valence electrons given by one oxygen atom = 4 + 6 = 10.
Step 2 Identify the central atom
The determination of the central atom in polyatomic compounds is based on the fact that the central atom must have a high valence electron or minimal electronegativity. However, since there are only two atoms in the CO molecule, any atom can be chosen as the central atom. It is only necessary that the two be connected by a single bond.
Step 3 Labelling the electron lone pairs between atoms
Total valance electrons pairs = σ bonds + π bonds + lone pairs at valence shells, total electron pairs are determined by dividing the number total valence electrons by two. For the CO molecule, the total number of electron pairs is 5.
The carbon atom is connected to the oxygen atom by a σ-bond (one σ-bond equals one electron pair), and the remaining four electron pairs are distributed as follows: one electron pair on the carbon atom and three electron pairs on the oxygen atom.
Step 4 Stability of structure and minimize charges on atoms by converting lone pairs to bonds
When there are positive and negative charges on lot of atoms or higher charges (like +2, +3, -2, -3) on atoms in an ion or molecule, that structure is not stable. Therefore, We should try to reduce charges on atoms if it is a possible.
To make the structure more stable we have to check if it is forming an octet, if it does not have an octet then the lone pair of electrons will move to form a double or triple bond, in order for this carbon atom to be stable the pair of electrons has to move away from the oxygen atom in the outer layer so that the carbon atom can have 8 electrons (i.e. an octet). After the move, a triple bond is formed between the carbon and oxygen atoms to meet the octet of each atom, while still using the 10 valence electrons available to the CO molecule.
);