How to use anisole correctly
Introduction
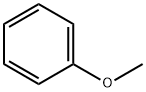
Anisole is an organic compound with the chemical formula C7H8O with a pleasant anise-like aroma, used in organic synthesis and also as a solvent, fragrance and insect repellent. For organic synthesis, it is also used as solvent, fragrance and insect repellent[1].

Picture 1 The physical map of anisole
Toxicological data
Anisole has acute toxicity, LD50: 3700mg/kg (oral in rats); 2800mg/kg (oral in mice). Rabbit percutaneous: 500mg (24h), moderate stimulation. Anisole is also mutagenic, causing DNA inhibition, 25 μmol/L in human lymphocytes.
Ecological data
The biodegradability of anisole was tested by MITI, the initial concentration was 100 ppm, and the degradation was 56% after 2 weeks, and the BOD removal rate continued to increase. To test the non-biodegradability of anisole in air, when the hydroxyl radical concentration is 5.00×105/cm3, the degradation half-life is 22h.
First-aid
In the event of skin contact with anisole, remove contaminated clothing and rinse skin thoroughly with soap and water. In case of eye contact with anisole, lift the eyelids, rinse with running water or normal saline and seek medical attention in time. If anisole is accidentally inhaled, it should be quickly removed from the scene to fresh air. Keep the airway open. If breathing is difficult, give oxygen. If breathing stops, give artificial respiration immediately. seek medical attention. If anisole is accidentally ingested, drink plenty of warm water, induce vomiting and seek medical attention immediately.
Fire-fighting measures
The burning of anisole will produce harmful substances, mostly carbon monoxide and carbon dioxide.
Extinguishing methods are to cool the container with water spray and move the container from the fire to an open area if possible. Extinguishing agents are foam, carbon dioxide, dry powder, and sand. Fire extinguishing with water is ineffective.
Emergency release treatment
Quickly evacuate personnel from the leaked contaminated area to a safe area, isolate them, and strictly restrict access. Cut off the source of ignition. It is recommended that emergency personnel wear self-contained positive pressure breathing apparatus and anti-static overalls. Cut off sources of leaks as much as possible. Prevent flow into restricted spaces such as sewers and flood drains. Absorb or absorb with sand or other non-combustible materials in the event of a small spill. It can also be scrubbed with an emulsion made of a non-flammable dispersant, which is diluted and put into the waste water system. Build embankments or dig pits for containment in the event of a large spill. Cover with foam to reduce vapor hazards. Transfer it to a tanker or a special collector with an explosion-proof pump, and recycle it or transport it to a waste disposal site for disposal.
Synthesis
Dimethyl carbonate was used instead of dimethyl sulfate as methylating agent, and anisole was synthesized by gas-phase reaction with phenol on NaX molecular sieve catalyst; and a corresponding small-scale device was self-made to obtain the best reaction of this new process. Conditions: The mass ratio of phenol to dimethyl carbonate is 1:1, the reaction temperature is 280 °C, the average residence time is 6 s, and the yield of anisole can reach 84-3%.
The traditional production process of anisole is seriously polluted, and the development of efficient and pollution-free production process is very important for environmental protection. In this paper, the research progress in the synthesis of anisole with dimethyl carbonate and methanol as methylation reagents is reviewed, and the catalytic activities of inorganic salts, phase transfer catalysts, ionic liquids, transition metal complexes, etc. are compared, and anisole The development trend of synthesis technology is prospected.
Handling and storage
Operation precautions: closed operation, full ventilation. Operators must undergo special training and strictly abide by operating procedures. It is recommended that operators wear filter respirators (half masks), chemical safety goggles, anti-static work clothes, and rubber oil-resistant gloves. Keep away from fire and heat sources, and smoking is strictly prohibited in the workplace. Use explosion-proof ventilation systems and equipment. Prevent vapors from leaking into the workplace air. Avoid contact with oxidants and acids. Filling should control the flow rate to prevent the accumulation of static electricity. When handling, it should be lightly loaded and unloaded to prevent damage to packaging and containers. Equipped with the corresponding variety and quantity of fire fighting equipment and leakage emergency treatment equipment. Empty containers may be harmful residues.
Storage Precautions: Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 30℃. Keep container tightly closed. It should be stored separately from oxidants and acids, and should not be mixed. Use explosion-proof lighting and ventilation facilities. Prohibit the use of mechanical equipment and tools that are prone to sparks. Storage areas should be equipped with emergency release equipment and suitable containment materials.
Reference
1 Seip H M, Seip R. On the Structure of Gaseous Anisole[J]. Acta chem. scand, 1973, 27: 4024-4027.
);You may like
See also
Lastest Price from Anisole manufacturers

US $5.00-1.00/kg2024-04-28
- CAS:
- 100-66-3
- Min. Order:
- 1kg
- Purity:
- 99.5
- Supply Ability:
- 50 ton per month

US $10.00/kg2024-04-26
- CAS:
- 100-66-3
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 1000000000 ton