Industrial production method of dicyclopentadiene
Background
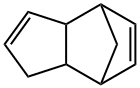
Dicyclopentadiene (C10H12) is a dimer of cyclopentadiene with a boiling point of 170°C, a melting point of 315°C and a density of 0.979[1]. There are two isomers of bridged ring type and hanging ring type in the space structure[1]. At room temperature, cyclopentadiene dimerizes to form a bridged ring type, and when heated to a high temperature of 150℃, it dimerizes to form a hanging ring type. Bridged cyclic dicyclopentadiene is mainly used in industry. High-purity dicyclopentadiene is a colorless crystal at room temperature. When it contains impurities, it is a light yellow oily liquid with a pungent camphor smell. It is insoluble in water and soluble in organic solvents such as alcohol and ether. Because dicyclopentadiene contains multiple unsaturated double bonds, its chemical properties are very active, and it can react with a variety of compounds to generate a wide variety of derivatives, which can be used in ethylene-propylene rubber, unsaturated polyester, synthetic petroleum resin, synthetic coating resin , Binder resin, papermaking filler resin, medicine and fuel, synthetic materials, synthetic fragrances, organic chemical intermediates, etc. production, dicyclopentadiene can also be depolymerized into cyclopentadiene, cyclopentadiene is a synthetic resin, Intermediates of pesticides, medicines, spices, etc.
Industrial production method of dicyclopentadiene
Dicyclopentadiene (DCPD) was obtained from coal tar in the early days, and the quantity is very small, so it has not been paid much attention[2]. Since then, because it can be recovered from the C5 component of petroleum cracking products, the source is abundant and the price is greatly reduced, so DCPD has attracted extensive attention. In the early 1980s, DCPD was introduced into resin synthesis. After continuous research and development, it has developed into a large series of unsaturated polyester resin products, which are widely used in corrosion resistance, high temperature resistance, marine, surface coating, SMC/ BMC and other fields.
Dicyclopentadiene petroleum resin is usually obtained by polymerizing dicyclopentadiene under high temperature and high pressure. Dicyclopentadiene petroleum resin usually has the characteristics of high softening point, good tackifying effect and strong stability. Its biggest use is in the fields of rubber mixing and ink additives, and can also be used in paint coatings and adhesives. However, at present, the domestic application of dicyclopentadiene in the synthesis of dicyclopentadiene resin is less, and more dicyclopentadiene is used in the production of unsaturated polyester resin and ethylene propylene rubber, and the synthesis of dicyclopentadiene resin is foreign. The main use of dicyclopentadiene.
Dicyclopentadiene was obtained from coal tar in the past in small quantities, so it was not taken seriously. With the development of the petroleum industry, the petroleum cracking fraction has developed from the initial mixed utilization to the separation and purification utilization. For the C5 fraction, it is mainly to separate and utilize the higher content of dienes (including: cyclopentadiene, isoprene and piperylene). Due to the special molecular structure and active chemical properties of these diolefins, many high value-added products can be synthesized, and they are valuable resources for chemical utilization. At the same time, because the by-product cyclopentadiene is very abundant, about 50 kt a-1 can be produced. For a set of 300kta-1 ethylene plant, the potential resource of dicyclopentadiene reaches 7~9kta-1, so the resources are more and more, and the price is greatly reduced.
Separation and purification method of dicyclopentadiene
There are two methods for separation and purification of dicyclopentadiene: thermal dimerization-depolymerization-distillation method and solvent extraction method. The thermal dimerization-depolymerization-distillation method is difficult to obtain high-purity cyclopentadiene products by common separation methods, and usually the cyclopentadiene in the C5 fraction is separated in the form of dicyclopentadiene. The extraction and rectification of C5 fraction by thermal dimerization can simultaneously separate the most valuable three kinds of dienes, such as cyclopentadiene, isoprene and piperylene in the C5 fraction. The solvent extraction method uses dimethylformamide (DMF) as the solvent to separate high-purity dicyclopentadiene and isoprene by using the relative volatility of dicyclopentadiene, piperylene and isoprene. and piperylene products. The method adopts one thermal dimerization, two extractive distillation and two rectification, and can separate three kinds of diolefins at the same time.
Application
Since DCPD can be converted into CPD at a certain temperature, the application of DCPD is greatly broadened. From basic organic chemical raw materials to new metal polymer materials, DCPD has important uses, and can be used as a catalyst for the production of unsaturated resins, metallocenes, adamantane, glutaraldehyde, dicyclopentadiene chloride (insecticide), etc. Raw materials, widely used in medicine, pesticides, spices, leather, synthetic rubber and other fields, and can also be used as high-energy fuel.
Reference
1 Hou Changli. Application Comparison of Dicyclopentadiene Resin and C9 Petroleum Resin: Science and Technology Innovation Review, 2013: 5: 133-134.
2 Wang Wei, Liu Jichun, Zhang Yuqing, Song Wensheng. New research progress of dicyclopentadiene: Chemistry and Adhesion, 2007: 02: 117-122.
);You may like
Lastest Price from Dicyclopentadiene manufacturers

US $0.00/Kg/Drum2024-04-26
- CAS:
- 77-73-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 5000mt
US $800.00/T2024-04-09
- CAS:
- 77-73-6
- Min. Order:
- 1T
- Purity:
- 80~85%
- Supply Ability:
- 1000T