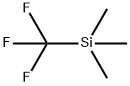
Instroduction of (Trifluoromethyl)trimethylsilane
General description
(Trifluoromethyl)trimethylsilane belong to organic fluorine chemistry has made rapid progress in recent decades, and the research and development of fluorine-containing compounds have had a great impact on our lives. Fluorine is a very special chemical element. Most organic fluorine compounds have some magical or even unimaginable properties. Therefore, it is used in many cutting-edge science and technology, industrial projects, pesticides, medicine, etc., and fluorine-containing compounds are used as a important development project has been actively researched and developed.[1]
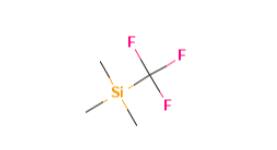
Figure 1 the molecular formula of (Trifluoromethyl)trimethylsilane
Application
1.The trifluoromethyl group is widely prevalent in many pharmaceuticals and agrochemicals because its incorporation into drug candidates could enhance chemical and metabolic stability, improve lipophilicity and bioavailability, and increase the protein bind affinity. Consequently, extensive attention has been devoted toward the development of efficient and versatile methods for introducing the CF3group into various organic molecules. Direct trifluoromethylation reaction has become one of the most efficient and important approaches for constructing carbon CF3bonds. Traditionally, the nucleophilic trifluoromethylation reaction involves an electrophile and the CF3anion, while the electrophilic trifluoromethylation reaction involves a nucleophile and the CF3cation. In 2010, we proposed the concept of oxidative trifluoromethylation: the reaction of nucleophilic substrates and nucleophilic trifluoromethylation reagents in the presence of oxidants[2]
2.The fluoride anion-initiated reaction of phenyl aromatic carboxylates with (trifluoromethyl)trimethylsilane (Me3SiCF3) with the formation of O-silyl-protected 2-aryl-1,1,1,3,3,3-hexafluoroisopropanols is reported. A phenoxide anion, generated during the trifluoromethylation of the phenyl carboxylate, functions to activate the Me3SiCF3, which permits a catalytic amount of the fluoride anion source to be used. Various functional groups, which can be used for further elaboration, are tolerated in the reaction.[3].Trifluoromethylation and Trifluoromethylthiolation Reactions Using (Trifluoromethyl)trimethylsilane as a Nucleophilic CF3 Source.
3.the application of synthesis
Oxidative trifluoromethylation reactions of various nucleophiles with CF3SiMe3 in the presence of oxidants.It focused most of our efforts on constructing carbon−CF3bonds via direct trifluoromethylation of various C−H bonds. This have demonstrated copper-mediated or -catalyzed or metal-free oxidative C−H trifluoromethylation of terminal alkynes, tertiary amines, arenes and heteroarenes, and terminal alkenes. Besides various C−H bonds, aryl boronic acids proved to be viable nucleophilic coupling partners for copper-mediated or -catalyzed cross-coupling reactions with CF3SiMe3. To further expand the reaction scope, we also applied H-phosphonates to the oxidative trifluoromethylation system to construct P−CF3bonds. Most recently, it developed silver-catalyzed hydrotrifluoromethylation of unactivated olefins. These studies explore boronic acids, C−H bonds, and P−H bonds as novel nucleophiles in transition-metal-mediated or -catalyzed cross-coupling reactions with CF3SiMe3, opening new viewpoints for future trifluoromethylation reactions. Furthermore, this also achieved the oxidative trifluoromethylthiolation reactions of aryl boronic acids and terminal alkynes to construct carbon−SCF3bonds by using CF3SiMe3and elemental sulfur as the nucleophilic trifluoromethylthiolating reagent.
Cyclopropenes that contain a silyl substituent on the alkene are important synthetic intermediates. For example, they can serve as precursors to allenylsilanes through photochemical rearrangement.Trimethylsilyl groups can efficiently control the regioselectivity of transformations such as cyclopropene carbometalation and Pauson–Khand reactions.Since silyl groups are readily removed from the double bond of cyclopropenes with fluoride or hydroxide/alkoxide ions, they can also serve as protecting groups for the olefin protons. Common methods used to construct 1- or 2-silyl-substituted cyclopropenes include cyclopropenation of an alkynylsilane eqn (1), and silylation of acyclopropenyl lithium species eqn (2).[4]
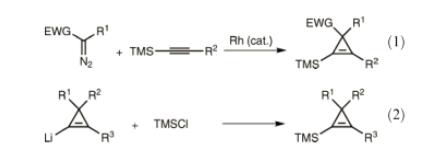
Figure 2 Copper-catalyzed silylation of cyclopropenes using (trifluoromethyl)trimethylsilane
Storage and Safty
(Trifluoromethyl)trimethylsilane should be storef in a cool, ventilated warehouse. Keep away from fire and heat sources. The packaging is required to be sealed and not in contact with air. It should be stored separately from oxidants, acids, and edible chemicals, and avoid mixed storage.
);You may like
Related articles And Qustion
Lastest Price from (Trifluoromethyl)trimethylsilane manufacturers

US $30.00/kg2024-04-26
- CAS:
- 81290-20-2
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 2000kg
US $0.10-0.10/kg2024-04-15
- CAS:
- 81290-20-2
- Min. Order:
- 0.004999999888241291kg
- Purity:
- ≥99.99%
- Supply Ability:
- 20 tons