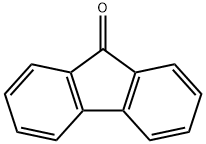
Is Fluorene nonpolar?
Fluorene is a colorless crystalline solid with a distinctive sweet odor, and it is insoluble in water. However, it is soluble in many organic solvents.It is commonly used in the manufacturing of OLEDs, dyes, and plasticizers.

The polarity of Fluorene
The polarity of a molecule is determined by the difference in electronegativity between the atoms that make up the molecule. Electronegativity is the measure of an atom's ability to attract electrons towards itself. If the difference in electronegativity between two atoms is small, the bond between them is considered nonpolar. On the other hand, if the difference is large, the bond is considered polar.
As to the fluorenone polarity,it is a hydrocarbon that consists of only carbon and hydrogen atoms. Carbon and hydrogen have very similar electronegativity values, so the bonds between them are nonpolar. Therefore, fluorene is considered a nonpolar molecule.
Fluorenone, on the other hand, has a polar C=O bond. The electronegativity of oxygen is much higher than that of carbon, so the bond between them is polar. As a result, fluorenone is more polar than fluorene.
);You may like
Related articles And Qustion
See also
Lastest Price from 9-Fluorenone manufacturers

US $0.00/kg2024-04-30
- CAS:
- 486-25-9
- Min. Order:
- 1kg
- Purity:
- 99.5%
- Supply Ability:
- 500mt

US $80.00/kg2024-02-27
- CAS:
- 486-25-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100 tons