Is glucose a polar molecule?
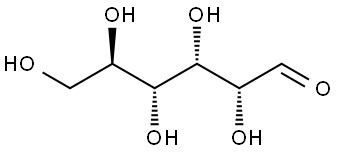
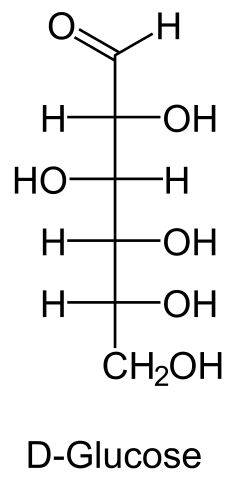
Glucose, commonly known as blood sugar, is represented by the chemical formula C6H12O6. It is a carbohydrate and a monosaccharide i.e., a simple sugar unit that is the main source of energy for the human body.
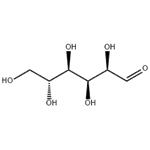
Glucose (C6H12O6) is a polar molecule. It is a hexose sugar containing multiple hydroxyl (OH) functional groups.The polarity of glucose arises from the presence of multiple hydroxyl (OH) groups that are attached to its carbon skeleton.
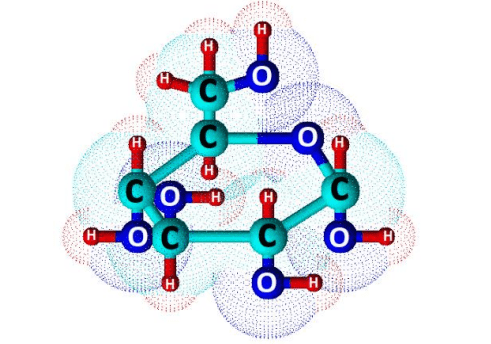
Glucose is a six-carbon compound, and consists of five hydroxyls (OH) functional groups and an aldehyde (CHO) group at carbon number 1. The O-H bonds and a C=O bond are strongly polar, having an electronegativity difference of 1.24 units and 0.89 units between the covalently-bonded atoms, respectively.
Sugar is polar like water, so sugar molecules also have positive and negative ends. The polarity of glucose makes it highly soluble in water.
The ability of glucose to dissolve in water is essential for its biological functions.
In summary, glucose is a polar molecule due to its multiple hydroxyl groups, which create polar bonds. The polarity of glucose plays a essential role in biological processes such as energy metabolism.
);You may like
Lastest Price from D(+)-Glucose manufacturers

US $20.00-15.00/kg2024-04-28
- CAS:
- 50-99-7
- Min. Order:
- 1000kg
- Purity:
- 99%
- Supply Ability:
- 5000kg/week
US $6.00/kg2024-04-28
- CAS:
- 50-99-7
- Min. Order:
- 1kg
- Purity:
- More than 99%
- Supply Ability:
- 2000KG/Month